J Korean Med Sci.
2023 Oct;38(41):e335. 10.3346/jkms.2023.38.e335.
Far-Infrared Irradiation Decreases Proliferation in Basal and PDGFStimulated VSMCs Through AMPKMediated Inhibition of mTOR/p70S6K Signaling Axis
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Yeungnam University, Daegu, Korea
- 2AbT R&D Center, AZothBio Inc., Hanam, Korea
- KMID: 2547178
- DOI: http://doi.org/10.3346/jkms.2023.38.e335
Abstract
- Background
Far-infrared (FIR) irradiation has been reported to improve diverse cardiovascular diseases, including heart failure, hypertension, and atherosclerosis. The dysregulated proliferation of vascular smooth muscle cells (VSMCs) is well established to contribute to developing occlusive vascular diseases such as atherosclerosis and in-stent restenosis. However, the effects of FIR irradiation on VSMC proliferation and the underlying mechanism are unclear. This study investigated the molecular mechanism through which FIR irradiation inhibited VSMC proliferation.
Methods
We performed cell proliferation and cell death assay, adenosine 5′-triphosphate (ATP) assay, inhibitor studies, transfection of dominant negative (dn)-AMP-activated protein kinase (AMPK) α1 gene, and western blot analyses. We also conducted confocal microscopic image analyses and ex vivo studies using isolated rat aortas.
Results
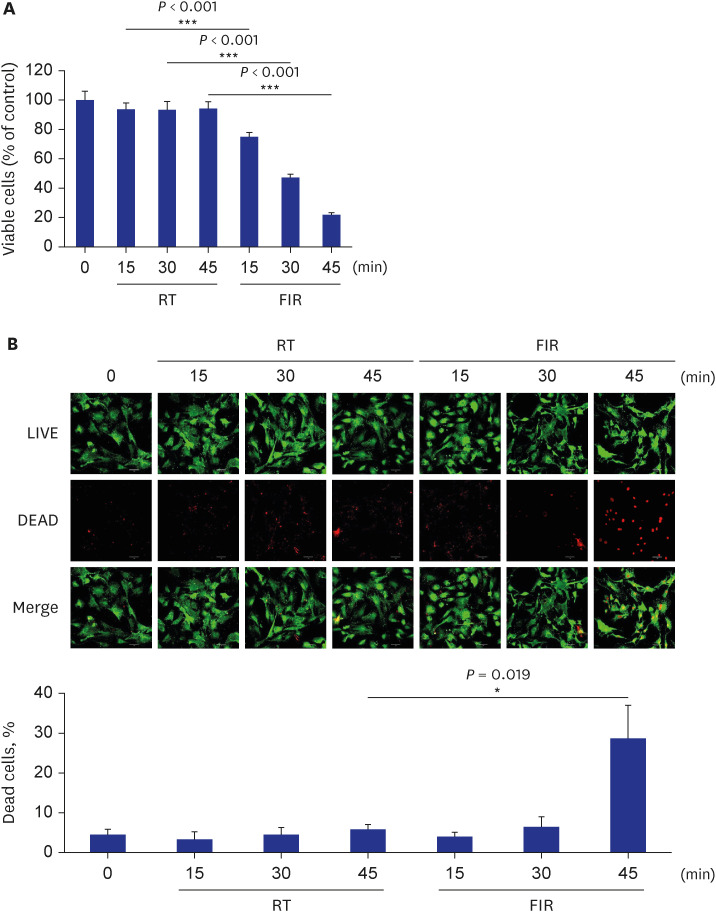
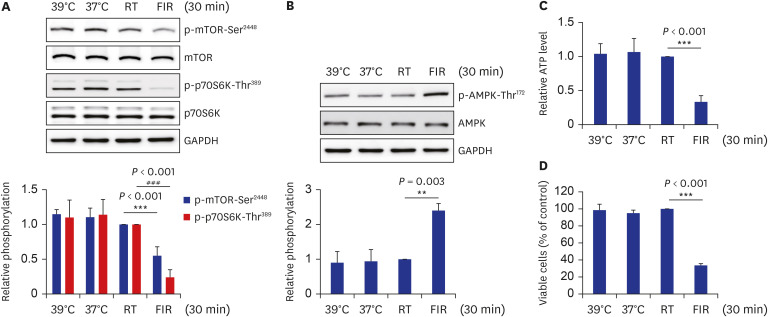
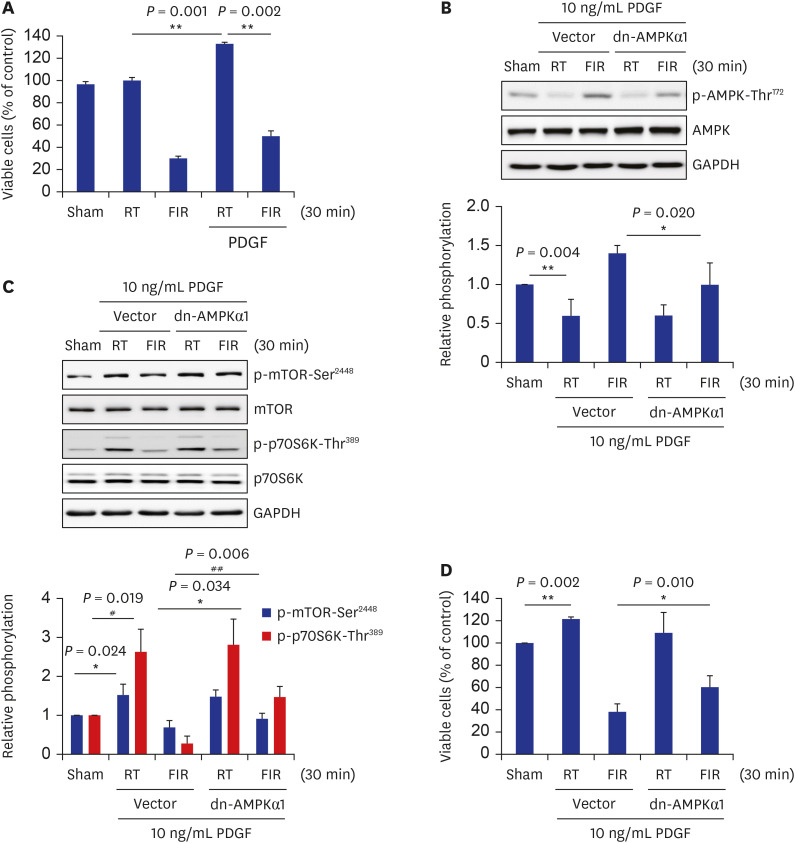
FIR irradiation for 30 minutes decreased VSMC proliferation without altering the cell death. Furthermore, FIR irradiation accompanied decreases in phosphorylation of the mammalian target of rapamycin (mTOR) at Ser2448 (p-mTOR-Ser2448 ) and p70 S6 kinase (p70S6K) at Thr389 (p-p70S6K-Thr389 ). The phosphorylation of AMPK at Thr172 (p-AMPKThr172 ) was increased in FIR-irradiated VSMCs, which was accompanied by a decreased cellular ATP level. Similar to in vitro results, FIR irradiation increased p-AMPK-Thr172 and decreased p-mTOR-Ser 2448 and p-p70S6K-Thr389 in isolated rat aortas. Pre-treatment with compound C, a specific AMPK inhibitor, or ectopic expression of dn-AMPKα1 gene, significantly reversed FIR irradiation-decreased VSMC proliferation, p-mTOR-Ser2448 , and p-p70S6K-Thr389 . On the other hand, hyperthermal stimulus (39°C) did not alter VSMC proliferation, cellular ATP level, and AMPK/mTOR/p70S6K phosphorylation. Finally, FIR irradiation attenuated plateletderived growth factor (PDGF)-stimulated VSMC proliferation by increasing p-AMPK-Thr172 , and decreasing p-mTOR-Ser2448 and p-p70S6K-Thr389 in PDGF-induced in vitro atherosclerosis model.
Conclusion
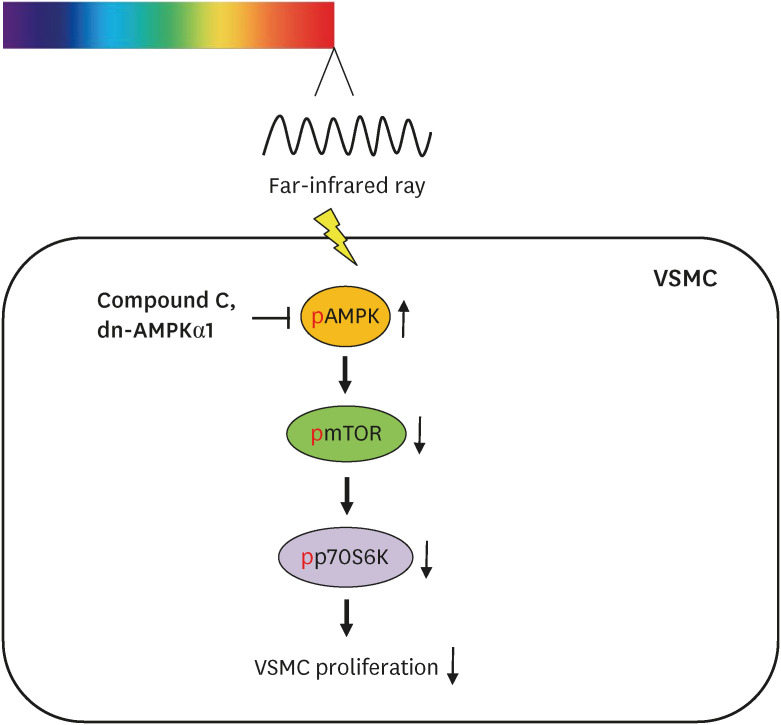
These results show that FIR irradiation decreases the basal and PDGF-stimulated VSMC proliferation, at least in part, by the AMPK-mediated inhibition of mTOR/p70S6K signaling axis irrespective of its hyperthermal effect. These observations suggest that FIR therapy can be used to treat arterial narrowing diseases, including atherosclerosis and in-stent restenosis.
Keyword
Figure
Reference
-
1. Vatansever F, Hamblin MR. Far infrared radiation (FIR): its biological effects and medical applications. Photonics Lasers Med. 2012; 4(4):255–266. PMID: 23833705.2. Hsu YH, Chen YC, Chen TH, Sue YM, Cheng TH, Chen JR, et al. Far-infrared therapy induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in human umbilical vein endothelial cells. PLoS One. 2012; 7(1):e30674. PMID: 22292015.3. Beever R. Far-infrared saunas for treatment of cardiovascular risk factors: summary of published evidence. Can Fam Physician. 2009; 55(7):691–696. PMID: 19602651.4. Shemilt R, Bagabir H, Lang C, Khan F. Potential mechanisms for the effects of far-infrared on the cardiovascular system - a review. Vasa. 2019; 48(4):303–312. PMID: 30421656.5. Masuda A, Miyata M, Kihara T, Minagoe S, Tei C. Repeated sauna therapy reduces urinary 8-epi-prostaglandin F(2alpha). Jpn Heart J. 2004; 45(2):297–303. PMID: 15090706.6. Lin CC, Chung MY, Yang WC, Lin SJ, Lee PC. Length polymorphisms of heme oxygenase-1 determine the effect of far-infrared therapy on the function of arteriovenous fistula in hemodialysis patients: a novel physicogenomic study. Nephrol Dial Transplant. 2013; 28(5):1284–1293. PMID: 23345623.7. Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev. 2017; 97(4):1555–1617. PMID: 28954852.8. Metz RP, Patterson JL, Wilson E. Vascular smooth muscle cells: isolation, culture, and characterization. Methods Mol Biol. 2012; 843:169–176. PMID: 22222531.9. Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, et al. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018; 114(4):529–539. PMID: 29394331.10. Basatemur GL, Jørgensen HF, Clarke MC, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019; 16(12):727–744. PMID: 31243391.11. Tang HY, Chen AQ, Zhang H, Gao XF, Kong XQ, Zhang JJ. Vascular smooth muscle cells phenotypic switching in cardiovascular diseases. Cells. 2022; 11(24):4060. PMID: 36552822.12. Nishida M, Tanaka T, Mangmool S, Nishiyama K, Nishimura A. Canonical transient receptor potential channels and vascular smooth muscle cell plasticity. J Lipid Atheroscler. 2020; 9(1):124–139. PMID: 32821726.13. Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY, Liao X, et al. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 2018; 14:656–668. PMID: 29175753.14. Kim J, Lee KP, Kim BS, Lee SJ, Moon BS, Baek S. Heat shock protein 90 inhibitor AUY922 attenuates platelet-derived growth factor-BB-induced migration and proliferation of vascular smooth muscle cells. Korean J Physiol Pharmacol. 2020; 24(3):241–248. PMID: 32392915.15. Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994; 331(8):489–495. PMID: 8041413.16. Hedin U, Roy J, Tran PK. Control of smooth muscle cell proliferation in vascular disease. Curr Opin Lipidol. 2004; 15(5):559–565. PMID: 15361792.17. Hillebrands JL, Rozing J. Chronic transplant dysfunction and transplant arteriosclerosis: new insights into underlying mechanisms. Expert Rev Mol Med. 2003; 5(2):1–23.18. Sarjeant JM, Rabinovitch M. Understanding and treating vein graft atherosclerosis. Cardiovasc Pathol. 2002; 11(5):263–271. PMID: 12361836.19. Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017; 66(6):789–800. PMID: 28622524.20. Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016; 48(7):e245. PMID: 27416781.21. Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012; 441(1):1–21. PMID: 22168436.22. Ai F, Chen M, Yu B, Yang Y, Xu G, Gui F, et al. Berberine regulates proliferation, collagen synthesis and cytokine secretion of cardiac fibroblasts via AMPK-mTOR-p70S6K signaling pathway. Int J Clin Exp Pathol. 2015; 8(10):12509-16. PMID: 26722438.23. Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003; 8(1):65–79. PMID: 12558800.24. Osman I, Segar L. Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem Pharmacol. 2016; 101:54–70. PMID: 26643070.25. Hwang YJ, Park JH, Cho DH. Activation of AMPK by telmisartan decreases basal and PDGF-stimulated VSMC proliferation via inhibiting the mTOR/p70S6K signaling axis. J Korean Med Sci. 2020; 35(35):e289. PMID: 32893519.26. Cho DH, Lee HJ, Lee JY, Park JH, Jo I. Far-infrared irradiation inhibits breast cancer cell proliferation independently of DNA damage through increased nuclear Ca2+/calmodulin binding modulated-activation of checkpoint kinase 2. J Photochem Photobiol B. 2021; 219:112188. PMID: 33901880.27. Hwang S, Lee DH, Lee IK, Park YM, Jo I. Far-infrared radiation inhibits proliferation, migration, and angiogenesis of human umbilical vein endothelial cells by suppressing secretory clusterin levels. Cancer Lett. 2014; 346(1):74–83. PMID: 24334140.28. Cho DH. Telmisartan inhibits nitric oxide production and vessel relaxation via protein phosphatase 2A-mediated endothelial NO synthase-Ser1179 dephosphorylation. J Korean Med Sci. 2019; 34(42):e266. PMID: 31674157.29. Hwang YJ, Cho DH. Activation of AMPK/proteasome/MLCK degradation signaling axis by telmisartan inhibits VSMC contractility and vessel contraction. Biochem Biophys Res Commun. 2020; 524(4):853–860. PMID: 32046856.30. Frismantiene A, Philippova M, Erne P, Resink TJ. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018; 52:48–64. PMID: 30172025.31. Baumann P, Mandl-Weber S, Emmerich B, Straka C, Schmidmaier R. Activation of adenosine monophosphate activated protein kinase inhibits growth of multiple myeloma cells. Exp Cell Res. 2007; 313(16):3592–3603. PMID: 17669398.32. Yu SY, Chiu JH, Yang SD, Hsu YC, Lui WY, Wu CW. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photomed. 2006; 22(2):78–86. PMID: 16606412.33. Hattori T, Kokura S, Okuda T, Okayama T, Takagi T, Handa O, et al. Antitumor effect of whole body hyperthermia with alpha-galactosylceramide in a subcutaneous tumor model of colon cancer. Int J Hyperthermia. 2007; 23(7):591–598. PMID: 18038289.34. Udagawa Y, Nagasawa H, Kiyokawa S. Inhibition by whole-body hyperthermia with far-infrared rays of the growth of spontaneous mammary tumours in mice. Anticancer Res. 1999; 19(5B):4125–4130. PMID: 10628363.35. Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004; 15(4):237–254. PMID: 15207815.36. Lee H, Hwang YJ, Park JH, Cho DH. Valproic acid decreases vascular smooth muscle cell proliferation via protein phosphatase 2A-mediated p70 S6 kinase inhibition. Biochem Biophys Res Commun. 2022; 606:94–99. PMID: 35339758.37. Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012; 95(2):156–164. PMID: 22406749.38. Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002; 8(11):1249–1256. PMID: 12411952.39. Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995; 77(3):445–465. PMID: 7641318.40. Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992; 326(4):242–250. PMID: 1727977.41. Hong MK. Restenosis following coronary angioplasty: current status. Korean J Intern Med. 2001; 16(2):51–55. PMID: 11590901.42. Yamamoto K, Ohishi M, Ho C, Kurtz TW, Rakugi H. Telmisartan-induced inhibition of vascular cell proliferation beyond angiotensin receptor blockade and peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009; 54(6):1353–1359. PMID: 19822796.43. Li Q, Peng J, Luo Y, Zhou J, Li T, Cao L, et al. Far infrared light irradiation enhances Aβ clearance via increased exocytotic microglial ATP and ameliorates cognitive deficit in Alzheimer’s disease-like mice. J Neuroinflammation. 2022; 19(1):145. PMID: 35701825.44. Chang JC, Wu SL, Hoel F, Cheng YS, Liu KH, Hsieh M, et al. Far-infrared radiation protects viability in a cell model of Spinocerebellar Ataxia by preventing polyQ protein accumulation and improving mitochondrial function. Sci Rep. 2016; 6(1):30436. PMID: 27469193.45. Hsu YH, Chen YC, Chen YW, Chiu TH, Kuo YT, Chen CH. Far-infrared radiation prevents decline in β-cell mass and function in diabetic mice via the mitochondria-mediated Sirtuin1 pathway. Metabolism. 2020; 104:154143. PMID: 31927009.46. Hsu YH, Chen YW, Cheng CY, Lee SL, Chiu TH, Chen CH. Detecting the limits of the biological effects of far-infrared radiation on epithelial cells. Sci Rep. 2019; 9(1):11586. PMID: 31406226.47. Dyla M, Basse Hansen S, Nissen P, Kjaergaard M. Structural dynamics of P-type ATPase ion pumps. Biochem Soc Trans. 2019; 47(5):1247–1257. PMID: 31671180.48. Smith IC, Bombardier E, Vigna C, Tupling AR. ATP consumption by sarcoplasmic reticulum Ca2+ pumps accounts for 40-50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PLoS One. 2013; 8(7):e68924. PMID: 23840903.49. Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci U S A. 1999; 96(8):4438–4442. PMID: 10200280.50. Petritsch C, Beug H, Balmain A, Oft M. TGF-beta inhibits p70 S6 kinase via protein phosphatase 2A to induce G(1) arrest. Genes Dev. 2000; 14(24):3093–3101. PMID: 11124802.51. Cho DH, Choi YJ, Jo SA, Ryou J, Kim JY, Chung J, et al. Troglitazone acutely inhibits protein synthesis in endothelial cells via a novel mechanism involving protein phosphatase 2A-dependent p70 S6 kinase inhibition. Am J Physiol Cell Physiol. 2006; 291(2):C317–C326. PMID: 16825603.52. Toyokawa H, Matsui Y, Uhara J, Tsuchiya H, Teshima S, Nakanishi H, et al. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp Biol Med (Maywood). 2003; 228(6):724–729. PMID: 12773705.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of AMPK by Telmisartan Decreases Basal and PDGF-stimulated VSMC Proliferation via Inhibiting the mTOR/p70S6K Signaling Axis
- The Inhibition of Insulin-stimulated Proliferation of Vascular Smooth Muscle Cells by Rosiglitazone Is Mediated by the Akt-mTOR-P70S6K Pathway
- Erratum to “The Inhibition of Insulin-stimulated Proliferation of Vascular Smooth Muscle Cells by Rosiglitazone Is Mediated by the Akt-mTOR-P70S6K Pathway” by Park S, et al. (Yonsei Med J 2008 Aug;49(4):592-600)
- Sulfatase 1 mediates the inhibitory effect of angiotensin II type 2 receptor inhibitor on angiotensin II-induced hypertensive mediator expression and proliferation in vascular smooth muscle cells from spontaneously hypertensive rats
- P70S6K and Elf4E Dual Inhibition Is Essential to Control Bladder Tumor Growth and Progression in Orthotopic Mouse Non-muscle Invasive Bladder Tumor Model