J Korean Med Sci.
2013 Sep;28(9):1362-1372. 10.3346/jkms.2013.28.9.1362.
Early Experience of Pre- and Post-Contrast 7.0T MRI in Brain Tumors
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- 2Department of Neurosurgery, Physiology, and Biomedical Engineering, Mayo Clinic, Mineapolis, MN, USA.
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Radiology, Seoul National University Hospital, Seoul, Korea.
- 6Neuroscience Research Institute, Gachon University of Medicine and Science, Incheon, Korea. zcho@gachon.ac.kr
- KMID: 1793054
- DOI: http://doi.org/10.3346/jkms.2013.28.9.1362
Abstract
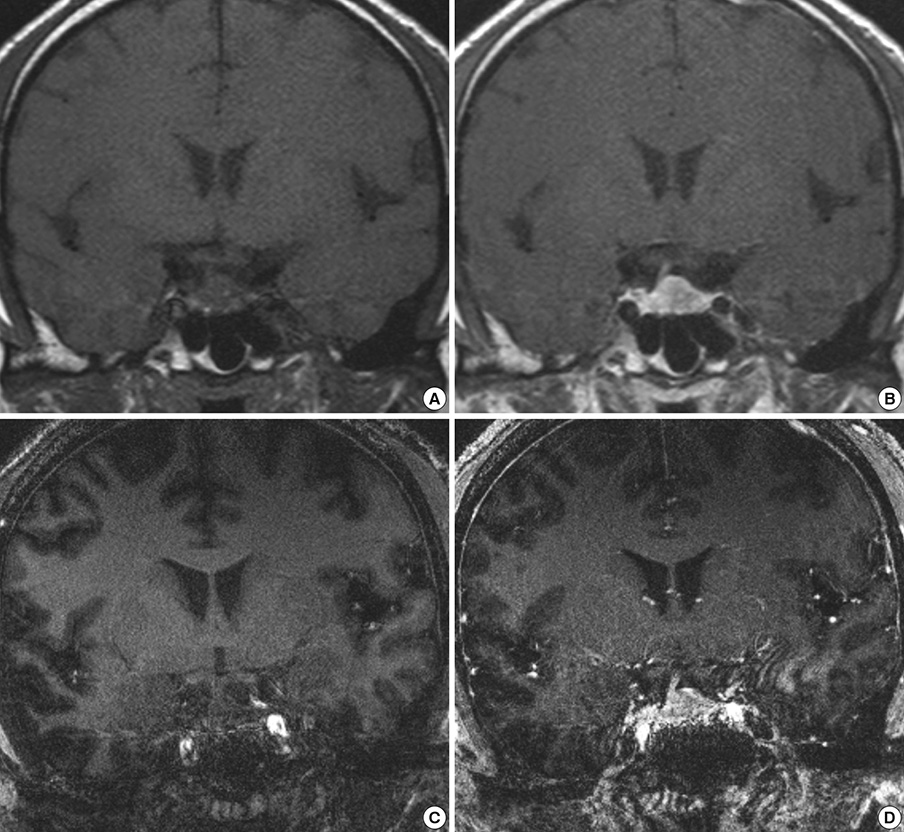
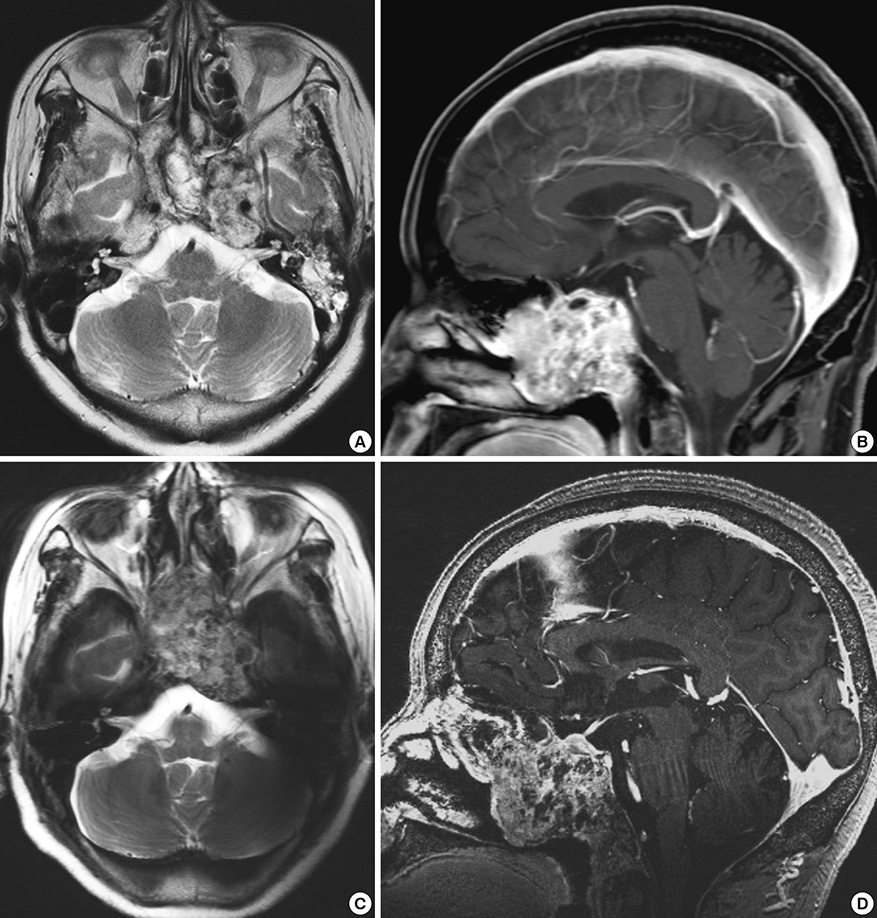
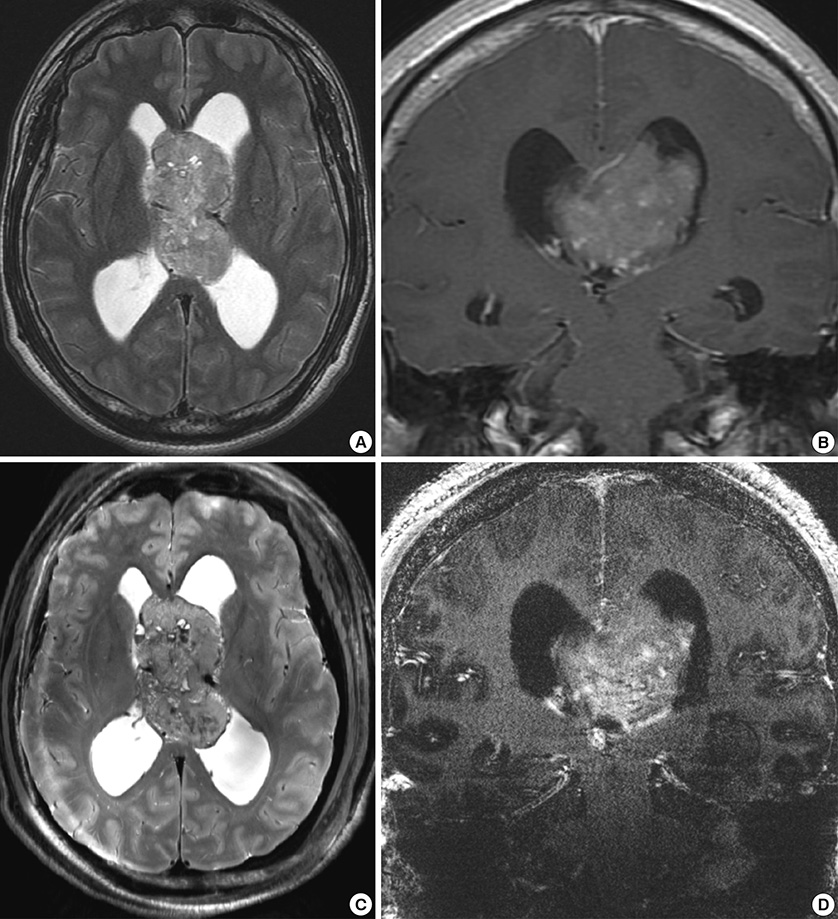
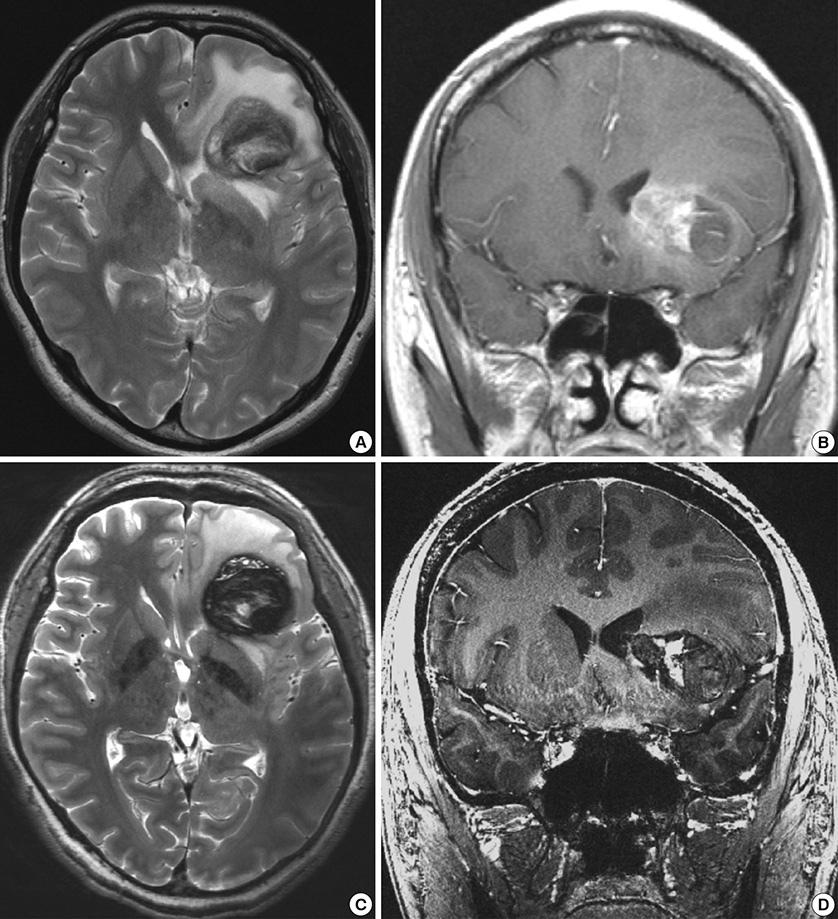
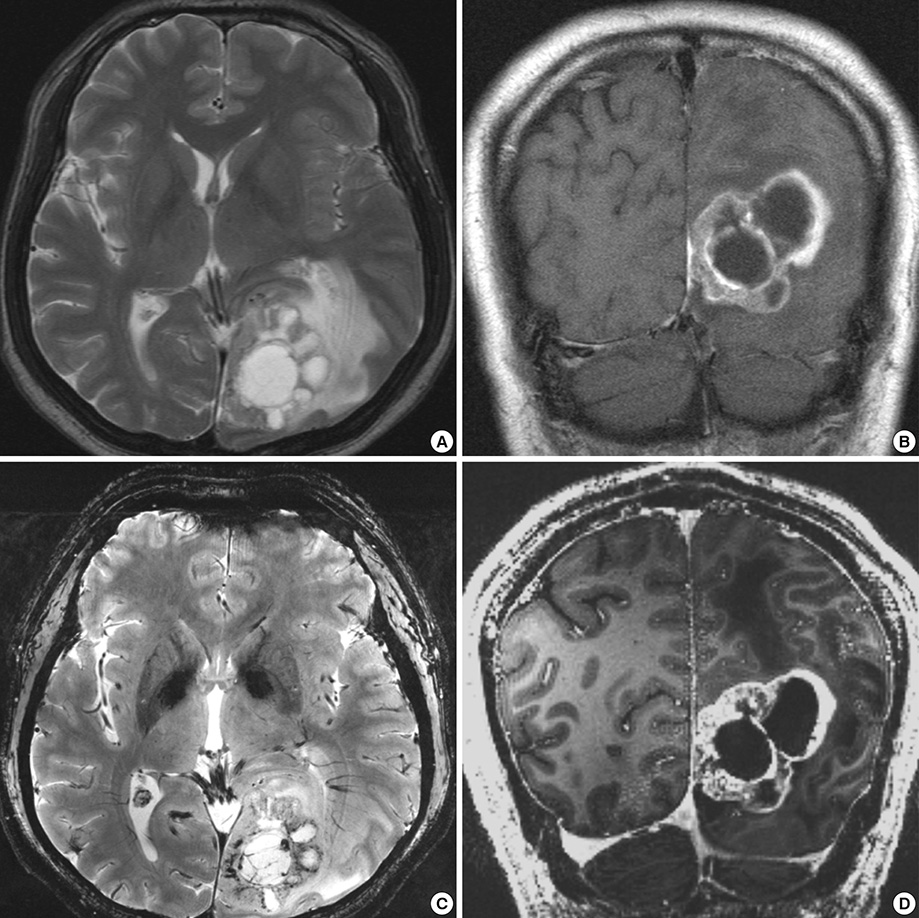
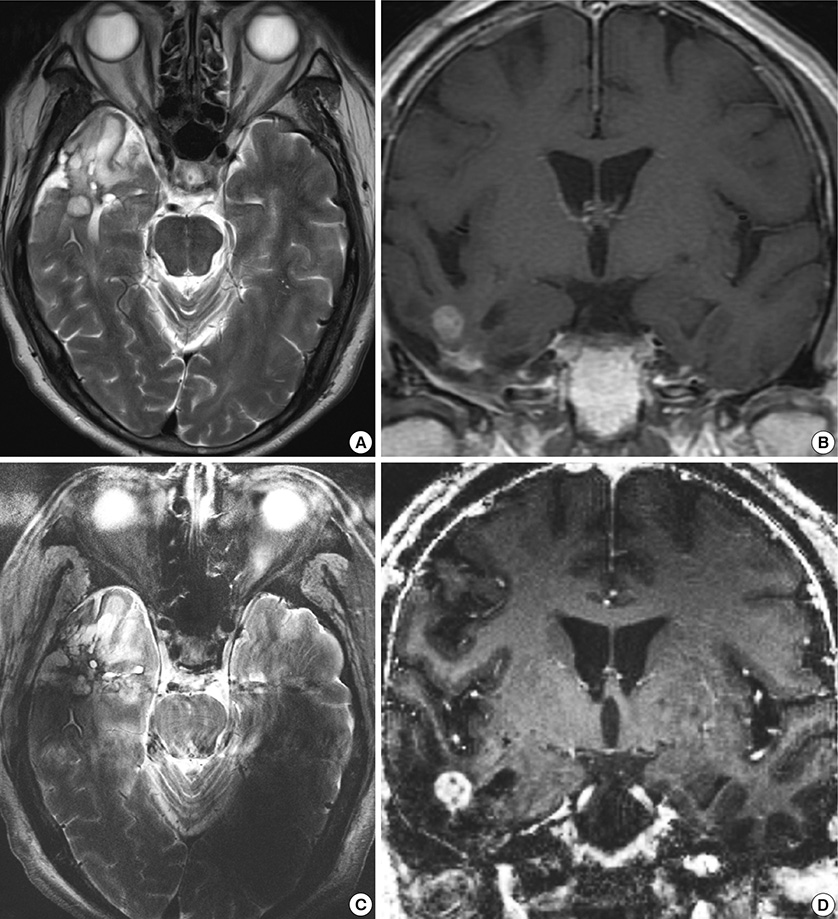
- We investigated the safety and clinical applicability of 7.0 Tesla (T) brain magnetic resonance imaging (MRI) in patients with brain tumors. Twenty-four patients with intraaxial or extraaxial brain tumors were enrolled in this study. 7.0T MRIs of T2*-weighted axial and T1-weighted coronal or sagittal images were obtained and compared with 1.5T brain MRIs. The T2*-weighted images from 7.0T brain MRI revealed detailed microvasculature and the internal contents of supratentorial brain tumors better than that of 1.5T brain MRI. For brain tumors located in parasellar areas or areas adjacent to major cerebral vessels, flow-related artifacts were exaggerated in the 7.0T brain MRIs. For brain tumors adjacent to the skull base, susceptibility artifacts in the interfacing areas of the paranasal sinus and skull base hampered the aquisition of detailed images and information on brain tumors in the 7.0T brain MRIs. This study shows that 7.0T brain MRI can provide detailed information on the intratumoral components and margins in supratentorial brain tumors. Further studies are needed to develop refined MRI protocols for better images of brain tumors located in the skull base, parasellar, and adjacent major cerebrovascular structures.
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of 3 and 7 Tesla Magnetic Resonance Imaging of Obstructive Hydrocephalus Caused by Tectal Glioma
Hyeong Cheol Moon, Hyeon-Man Baek, Young Seok Park
Brain Tumor Res Treat. 2016;4(2):150-154. doi: 10.14791/btrt.2016.4.2.150.
Reference
-
1. Van der Kolk AG, Hendrikse J, Zwanenburg JJ, Visser F, Luijten PR. Clinical applications of 7 T MRI in the brain. Eur J Radiol. 2013; 82:708–718.2. Lupo JM, Banerjee S, Hammond KE, Kelley DA, Xu D, Chang SM, Vigneron DB, Majumdar S, Nelson SJ. GRAPPA-based susceptibility-weighted imaging of normal volunteers and patients with brain tumor at 7 T. Magn Reson Imaging. 2009; 27:480–488.3. Moenninghoff C, Maderwald S, Theysohn JM, Kraff O, Ladd ME, El Hindy N, van de Nes J, Forsting M, Wanke I. Imaging of adult astrocytic brain tumours with 7 T MRI: preliminary results. Eur Radiol. 2010; 20:704–713.4. Pinker K, Noebauer-Huhmann IM, Stavrou I, Hoeftberger R, Szomolanyi P, Weber M, Stadlbauer A, Grabner G, Knosp E, Trattnig S. High-field, high-resolution, susceptibility-weighted magnetic resonance imaging: improved image quality by addition of contrast agent and higher field strength in patients with brain tumors. Neuroradiology. 2008; 50:9–16.5. Strzhizhovskĭ AD, Galaktionova GV. Proliferation of bone marrow cells upon exposure to constant magnetic fields of ultra-high strength. Tsitologiia. 1978; 20:717–720.6. Zmyślony M, Aniołczyk H, Bortkiewicz A. Exposure to VHF and UHF electromagnetic fields among workers employed in radio and TV broadcast centers: I. assessment of exposure. Med Pr. 2001; 52:321–327.7. Kangarlu A, Burgess RE, Zhu H, Nakayama T, Hamlin RL, Abduljalil AM, Robitaille PM. Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn Reson Imaging. 1999; 17:1407–1416.8. Shrivastava D, Abosch A, Hanson T, Tian J, Gupte A, Iaizzo PA, Vaughan JT. Effect of the extracranial deep brain stimulation lead on radiofrequency heating at 9.4 Tesla (400.2 MHz). J Magn Reson Imaging. 2010; 32:600–607.9. Mansfield P, Bowley RM, Haywood B. Controlled E-field gradient coils. MAGMA. 2003; 16:113–120.10. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.11. Yuh WT, Christoforidis GA, Koch RM, Sammet S, Schmalbrock P, Yang M, Knopp MV. Clinical magnetic resonance imaging of brain tumors at ultrahigh field: a state-of-the-art review. Top Magn Reson Imaging. 2006; 17:53–61.12. Dashner RA, Kangarlu A, Clark DL, RayChaudhury A, Chakeres DW. Limits of 8-Tesla magnetic resonance imaging spatial resolution of the deoxygenated cerebral microvasculature. J Magn Reson Imaging. 2004; 19:303–307.13. Christoforidis GA, Grecula JC, Newton HB, Kangarlu A, Abduljalil AM, Schmalbrock P, Chakeres DW. Visualization of microvascularity in glioblastoma multiforme with 8-T high-spatial-resolution MR imaging. AJNR Am J Neuroradiol. 2002; 23:1553–1556.14. Abduljalil AM, Kangarlu A, Yu Y, Robitaille PM. Macroscopic susceptibility in ultra high field MRI: II: acquisition of spin echo images from the human head. J Comput Assist Tomogr. 1999; 23:842–844.15. Burgess RE, Yu Y, Christoforidis GA, Bourekas EC, Chakeres DW, Spigos D, Kangarlu A, Abduljalil AM, Robitaille PM. Human leptomeningeal and cortical vascular anatomy of the cerebral cortex at 8 Tesla. J Comput Assist Tomogr. 1999; 23:850–856.16. Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, et al. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001; 46:24–30.17. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1:27–31.18. Abramovitch R, Meir G, Neeman M. Neovascularization induced growth of implanted C6 glioma multicellular spheroids: magnetic resonance microimaging. Cancer Res. 1995; 55:1956–1962.19. Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002; 61:215–225.20. Brem S, Cotran R, Folkman J. Tumor angiogenesis: a quantitative method for histologic grading. J Natl Cancer Inst. 1972; 48:347–356.21. Cho ZH, Kang CK, Han JY, Kim SH, Kim KN, Hong SM, Park CW, Kim YB. Observation of the lenticulostriate arteries in the human brain in vivo using 7.0T MR angiography. Stroke. 2008; 39:1604–1606.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case Report of Preoperative and Postoperative 7.0T Brain MRI in a Patient with a Small Cell Glioblastoma
- Contrast-enhanced Magnetic Resonance Imaging of Brain Metastases at 7.0T versus 1.5T: A Preliminary Result
- Abbreviated Breast Magnetic Resonance Imaging: Background, Evidence From Studies, and Future Considerations
- Review of Recent Advancement of Ultra High Field Magnetic Resonance Imaging: from Anatomy to Tractography
- Delayed Effect of Contrast Enhancement in Brain Tumors on MRI