J Korean Med Sci.
2014 Jul;29(7):1012-1017. 10.3346/jkms.2014.29.7.1012.
A Case Report of Preoperative and Postoperative 7.0T Brain MRI in a Patient with a Small Cell Glioblastoma
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Cancer Research Institute, Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea. paeksh@snu.ac.kr
- 2Department of Radiology, Seoul National University Hospital, Cancer Research Institute, Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Seoul National University Hospital, Cancer Research Institute, Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Neuroscience Research Institute, Gachon University of Medicine and Science, Incheon, Korea.
- KMID: 1789964
- DOI: http://doi.org/10.3346/jkms.2014.29.7.1012
Abstract
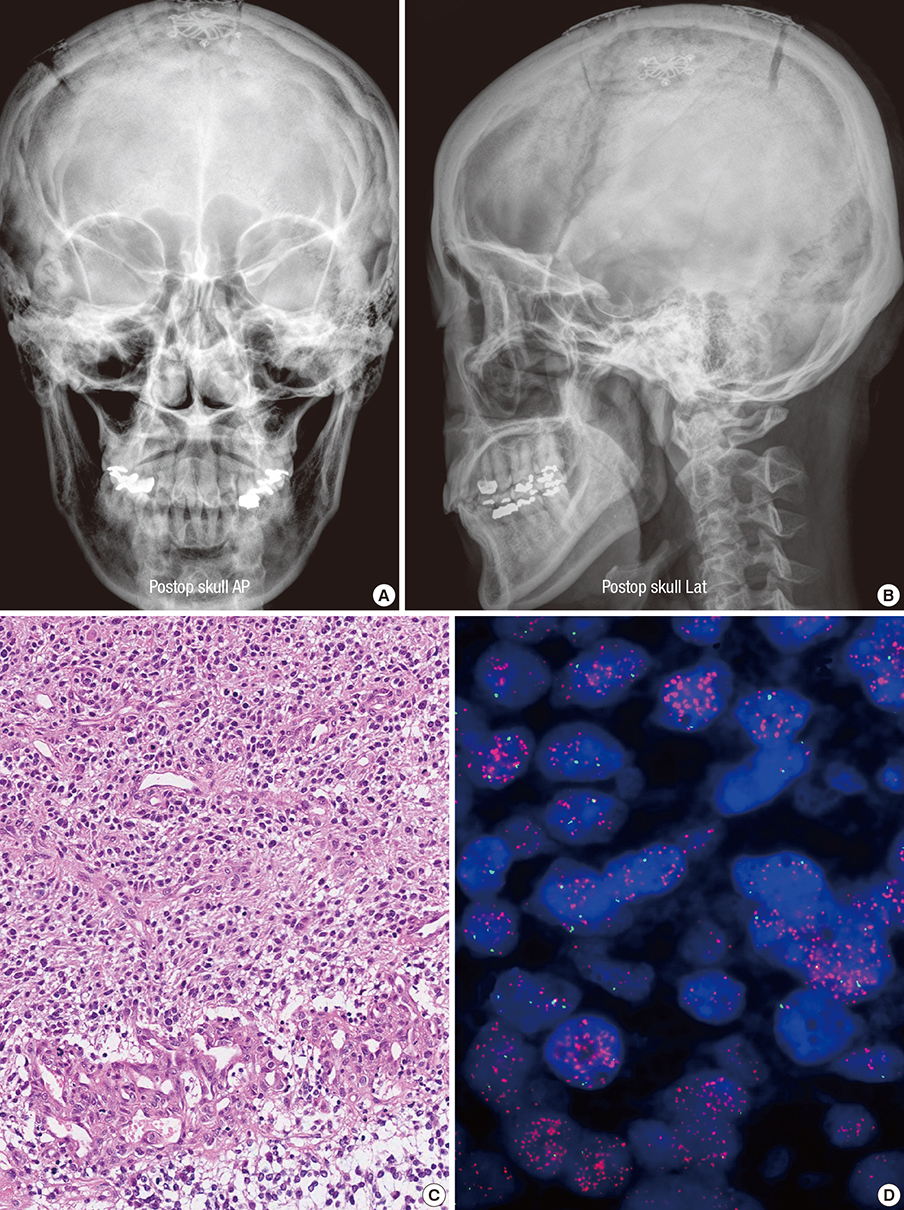
- A 45-yr-old female patient was admitted with one-month history of headache and progressive left hemiparesis. Brain magnetic resonance imaging (MRI) demonstrated a mass lesion in her right frontal lobe. Her brain tumor was confirmed as a small cell glioblastoma. Her follow-up brain MRI, taken at 8 months after her initial surgery demonstrated tumor recurrence in the right frontal lobe. Contrast-enhanced 7.0T brain magnetic resonance imaging (MRI) was safely performed before surgery and at the time of recurrence. Compared with 1.5T and 3.0T brain MRI, 7.0T MRI showed sharpened images of the brain tumor contexture with detailed anatomical information. The fused images of 7.0T and 1.5T brain MRI taken at the time of recurrence demonstrated no significant discrepancy in the positions of the anterior and the posterior commissures. It is suggested that 7.0T MRI can be safely utilized for better images of the maligant gliomas before and after surgery.
MeSH Terms
Figure
Reference
-
1. Abduljalil AM, Schmalbrock P, Novak V, Chakeres DW. Enhanced gray and white matter contrast of phase susceptibility-weighted images in ultra-high-field magnetic resonance imaging. J Magn Reson Imaging. 2003; 18:284–290.2. Thomas DL, De Vita E, Roberts S, Turner R, Yousry TA, Ordidge RJ. High-resolution fast spin echo imaging of the human brain at 4.7 T: implementation and sequence characteristics. Magn Reson Med. 2004; 51:1254–1264.3. Norris DG. High field human imaging. J Magn Reson Imaging. 2003; 18:519–529.4. Cha S, Johnson G, Wadghiri YZ, Jin O, Babb J, Zagzag D, Turnbull DH. Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: correlation with histopathology. Magn Reson Med. 2003; 49:848–855.5. Lupo JM, Banerjee S, Hammond KE, Kelley DA, Xu D, Chang SM, Vigneron DB, Majumdar S, Nelson SJ. GRAPPA-based susceptibility-weighted imaging of normal volunteers and patients with brain tumor at 7 T. Magn Reson Imaging. 2009; 27:480–488.6. Moenninghoff C, Maderwald S, Theysohn JM, Kraff O, Ladd ME, El Hindy N, van de Nes J, Forsting M, Wanke I. Imaging of adult astrocytic brain tumours with 7 T MRI: preliminary results. Eur Radiol. 2010; 20:704–713.7. Kollia K, Maderwald S, Putzki N, Schlamann M, Theysohn JM, Kraff O, Ladd ME, Forsting M, Wanke I. First clinical study on ultra-high-field MR imaging in patients with multiple sclerosis: comparison of 1.5T and 7T. AJNR Am J Neuroradiol. 2009; 30:699–702.8. Paek SL, Chung YS, Paek SH, Hwang JH, Sohn CH, Choi SH, Son YD, Kim YB, Kim DG, Lee KH, et al. Early experience of pre- and post-contrast 7.0T MRI in brain tumors. J Korean Med Sci. 2013; 28:1362–1372.9. Takeda T, Takeda A, Nagaoka T, Kunieda E, Takemasa K, Watanabe M, Hatou T, Oguro S, Katayama M. Gadolinium-enhanced three-dimensional magnetization-prepared rapid gradient-echo (3D MP-RAGE) imaging is superior to spin-echo imaging in delineating brain metastases. Acta Radiol. 2008; 49:1167–1173.10. Abduljalil AM, Robitaille PM. Macroscopic susceptibility in ultra high field MRI. J Comput Assist Tomogr. 1999; 23:832–841.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Glioblastoma Multiforme of the Cerebellum
- A Case of Multicentric Glioblastoma Multiforme
- Postoperative Radiation Therapy of Astrocytoma and Glioblastoma Multiforme
- Glioblastoma Mimicking Herpes Simplex Encephalitis
- Glioblastoma in a Patient with Neurofibromatosis Type 1: A Case Report and Review of the Literature