J Periodontal Implant Sci.
2012 Aug;42(4):136-143. 10.5051/jpis.2012.42.4.136.
Novel analysis model for implant osseointegration using ectopic bone formation via the recombinant human bone morphogenetic protein-2/macroporous biphasic calcium phosphate block system in rats: a proof-of-concept study
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. dentall@yuhs.ac
- 2Research Center on Materials of Biological Interest, Dental faculty, Nantes University, Nantes, France.
- 3Department of Orthopedics, Dongguk University Ilsan Hospital, Goyang, Korea.
- 4School of Chemical and Biological Engineering, Seoul National University, Seoul, Korea.
- KMID: 1783649
- DOI: http://doi.org/10.5051/jpis.2012.42.4.136
Abstract
- PURPOSE
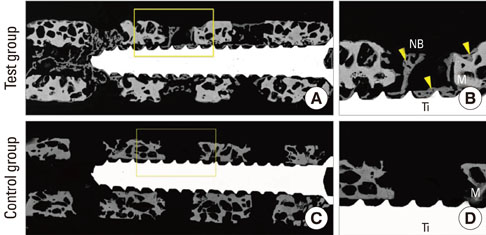
The osseointegration around titanium mini-implants installed in macroporous biphasic calcium phosphate (MBCP) blocks was evaluated after incubation with recombinant human bone morphogenetic protein-2 (rhBMP-2) in an ectopic subcutaneous rat model.
METHODS
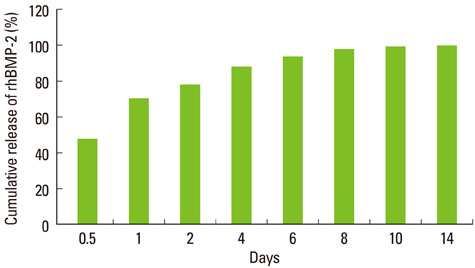
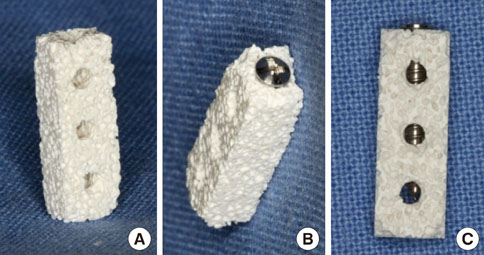
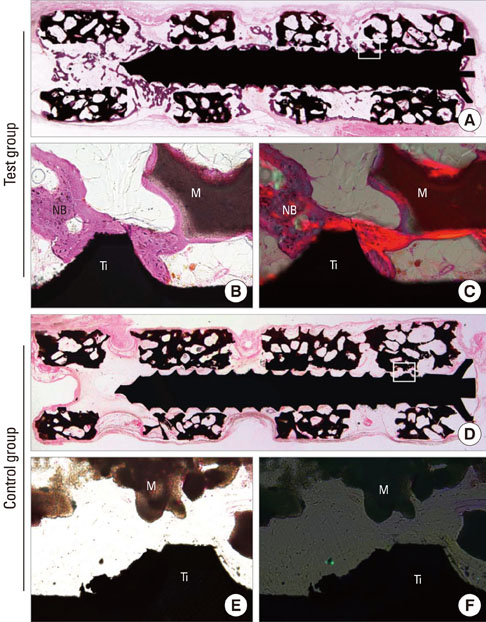
Mini-implants (phi1.8x12 mm) were installed in MBCP blocks (bMBCPs, 4x5x15 mm) loaded with rhBMP-2 at 0.1 mg/mL, and then implanted for 8 weeks into subcutaneous pockets of male Sprague-Dawley rats (n=10). A histomorphometric analysis was performed, and the bone-to-implant contact (BIC) and bone density were evaluated.
RESULTS
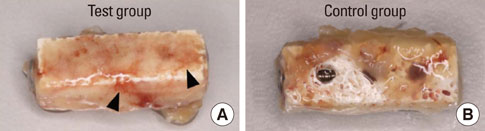
Significant osteoinductive activity was induced in the rhBMP-2/bMBCP group. The percentage of BIC was 41.23+/-4.13% (mean+/-standard deviation), while bone density was 33.47+/-5.73%. In contrast, no bone formation was observed in the bMBCP only group.
CONCLUSIONS
This model represents a more standardized tool for analyzing osseointegration and bone healing along the implant surface and in bMBCPs that excludes various healing factors derived from selected animals and defect models.
Keyword
MeSH Terms
-
Animals
Bone Density
Bone Morphogenetic Protein 2
Calcium
Dental Implants
Humans
Hydroxyapatites
Male
Nitrogen Mustard Compounds
Osseointegration
Osteogenesis
Rats
Rats, Sprague-Dawley
Recombinant Proteins
Titanium
Transforming Growth Factor beta
Bone Morphogenetic Protein 2
Calcium
Dental Implants
Hydroxyapatites
Nitrogen Mustard Compounds
Recombinant Proteins
Titanium
Transforming Growth Factor beta
Figure
Reference
-
1. Wikesjo UM, Guglielmoni P, Promsudthi A, Cho KS, Trombelli L, Selvig KA, et al. Periodontal repair in dogs: effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 1999. 26:392–400.
Article2. Blumenthal NM, Koh-Kunst G, Alves ME, Miranda D, Sorensen RG, Wozney JM, et al. Effect of surgical implantation of recombinant human bone morphogenetic protein-2 in a bioabsorbable collagen sponge or calcium phosphate putty carrier in intrabony periodontal defects in the baboon. J Periodontol. 2002. 73:1494–1506.
Article3. Boyne PJ, Nath R, Nakamura A. Human recombinant BMP-2 in osseous reconstruction of simulated cleft palate defects. Br J Oral Maxillofac Surg. 1998. 36:84–90.
Article4. Barboza EP, Duarte ME, Geolas L, Sorensen RG, Riedel GE, Wikesjo UM. Ridge augmentation following implantation of recombinant human bone morphogenetic protein-2 in the dog. J Periodontol. 2000. 71:488–496.
Article5. Jung JH, Yun JH, Um YJ, Jung UW, Kim CS, Choi SH, et al. Bone formation of Escherichia coli expressed rhBMP-2 on absorbable collagen block in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011. 111:298–305.
Article6. Jang JW, Yun JH, Lee KI, Jang JW, Jung UW, Kim CS, et al. Osteoinductive activity of biphasic calcium phosphate with different rhBMP-2 doses in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012. 113:480–487.
Article7. Kim CS, Kim JI, Kim J, Choi SH, Chai JK, Kim CK, et al. Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials. 2005. 26:2501–2507.
Article8. Jung RE, Glauser R, Scharer P, Hammerle CH, Sailer HF, Weber FE. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003. 14:556–568.
Article9. Smeets R, Maciejewski O, Gerressen M, Spiekermann H, Hanisch O, Riediger D, et al. Impact of rhBMP-2 on regeneration of buccal alveolar defects during the osseointegration of transgingival inserted implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. 108:e3–e12.
Article10. Wikesjo UM, Sorensen RG, Wozney JM. Augmentation of alveolar bone and dental implant osseointegration: clinical implications of studies with rhBMP-2. J Bone Joint Surg Am. 2001. 83:Suppl 1. (Pt 2):S136–S145.11. Nevins M, Kirker-Head C, Nevins M, Wozney JA, Palmer R, Graham D. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. Int J Periodontics Restorative Dent. 1996. 16:8–19.12. Lekholm U, Adell R, Lindhe J, Branemark PI, Eriksson B, Rockler B, et al. Marginal tissue reactions at osseointegrated titanium fixtures. (II) A cross-sectional retrospective study. Int J Oral Maxillofac Surg. 1986. 15:53–61.13. Hall J, Sorensen RG, Wozney JM, Wikesjo UM. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J Clin Periodontol. 2007. 34:444–451.
Article14. Herr G, Hartwig CH, Boll C, Kusswetter W. Ectopic bone formation by composites of BMP and metal implants in rats. Acta Orthop Scand. 1996. 67:606–610.
Article15. Wikesjo UM, Qahash M, Polimeni G, Susin C, Shanaman RH, Rohrer MD, et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol. 2008. 35:1001–1010.
Article16. Lee J, Decker JF, Polimeni G, Cortella CA, Rohrer MD, Wozney JM, et al. Evaluation of implants coated with rhBMP-2 using two different coating strategies: a critical-size supraalveolar peri-implant defect study in dogs. J Clin Periodontol. 2010. 37:582–590.
Article17. Susin C, Qahash M, Polimeni G, Lu PH, Prasad HS, Rohrer MD, et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-7 (rhBMP-7/rhOP-1): histological observations. J Clin Periodontol. 2010. 37:574–581.
Article18. Wikesjo UM, Susin C, Qahash M, Polimeni G, Leknes KN, Shanaman RH, et al. The critical-size supraalveolar peri-implant defect model: characteristics and use. J Clin Periodontol. 2006. 33:846–854.
Article19. Tatakis DN, Koh A, Jin L, Wozney JM, Rohrer MD, Wikesjo UM. Peri-implant bone regeneration using recombinant human bone morphogenetic protein-2 in a canine model: a dose-response study. J Periodontal Res. 2002. 37:93–100.
Article20. Decker JF, Lee J, Cortella CA, Polimeni G, Rohrer MD, Wozney JM, et al. Evaluation of implants coated with recombinant human bone morphogenetic protein-2 and vacuum-dried using the critical-size supraalveolar peri-implant defect model in dogs. J Periodontol. 2010. 81:1839–1849.
Article21. Becker J, Kirsch A, Schwarz F, Chatzinikolaidou M, Rothamel D, Lekovic V, et al. Bone apposition to titanium implants biocoated with recombinant human bone morphogenetic protein-2 (rhBMP-2). A pilot study in dogs. Clin Oral Investig. 2006. 10:217–224.
Article22. Jones AA, Buser D, Schenk R, Wozney J, Cochran DL. The effect of rhBMP-2 around endosseous implants with and without membranes in the canine model. J Periodontol. 2006. 77:1184–1193.
Article23. Langer R, Vacanti JP. Tissue engineering. Science. 1993. 260:920–926.
Article24. Park JC, So SS, Jung IH, Yun JH, Choi SH, Cho KS, et al. Induction of bone formation by Escherichia coli-expressed recombinant human bone morphogenetic protein-2 using block-type macroporous biphasic calcium phosphate in orthotopic and ectopic rat models. J Periodontal Res. 2011. 46:682–690.
Article25. Rohrer MD, Schubert CC. The cutting-grinding technique for histologic preparation of undecalcified bone and bone-anchored implants. Improvements in instrumentation and procedures. Oral Surg Oral Med Oral Pathol. 1992. 74:73–78.
Article26. Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS, Lee SH. In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A. 2008. 14:1285–1294.
Article27. Lee YJ, Jung SW, Chae GJ, Cho KS, Kim CS. The effect of recombinant human bone morphogenetic protein-2/macroporous biphasic calcium phosphate block system on bone formation in rat calvarial defects. J Korean Acad Periodontol. 2007. 37:Suppl. 397–407.
Article28. Lee JH, Kim CS, Choi KH, Jung UW, Yun JH, Choi SH, et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010. 31:3512–3519.
Article29. Bessho K, Konishi Y, Kaihara S, Fujimura K, Okubo Y, Iizuka T. Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg. 2000. 38:645–649.
Article30. Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol Lett. 2009. 31:1817–1824.
Article31. Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005. 16:329–345.
Article32. Kawakatsu N, Oda S, Kinoshita A, Kikuchi S, Tsuchioka H, Akizuki T, et al. Effect of rhBMP-2 with PLGA/gelatin sponge type (PGS) carrier on alveolar ridge augmentation in dogs. J Oral Rehabil. 2008. 35:647–655.
Article33. Schliephake H, Weich HA, Dullin C, Gruber R, Frahse S. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid: an experimental study in rats. Biomaterials. 2008. 29:103–110.
Article34. Le Nihouannen D, Saffarzadeh A, Gauthier O, Moreau F, Pilet P, Spaethe R, et al. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J Mater Sci Mater Med. 2008. 19:667–675.
Article35. Le Nihouannen D, Guehennec LL, Rouillon T, Pilet P, Bilban M, Layrolle P, et al. Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering. Biomaterials. 2006. 27:2716–2722.
Article36. Hong SJ, Kim CS, Han DK, Cho IH, Jung UW, Choi SH, et al. The effect of a fibrin-fibronectin/beta-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biomaterials. 2006. 27:3810–3816.
Article37. Abarrategi A, Moreno-Vicente C, Ramos V, Aranaz I, Sanz Casado JV, Lopez-Lacomba JL. Improvement of porous beta-TCP scaffolds with rhBMP-2 chitosan carrier film for bone tissue application. Tissue Eng Part A. 2008. 14:1305–1319.
Article38. Alam I, Asahina I, Ohmamiuda K, Enomoto S. Comparative study of biphasic calcium phosphate ceramics impregnated with rhBMP-2 as bone substitutes. J Biomed Mater Res. 2001. 54:129–138.
Article39. Oda S, Kinoshita A, Higuchi T, Shizuya T, Ishikawa I. Ectopic bone formation by biphasic calcium phosphate (BCP) combined with recombinant human bone morphogenetic protein-2 (rhBMP-2). J Med Dent Sci. 1997. 44:53–62.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Recombinant Human Bone Morphogenetic Protein-2/Macroporous Biphasic Calcium Phosphate Block system on Bone Formation in Rat Calvarial Defects
- rhBMP-2 using biphasic calcium phosphate block as a carrier induces new bone formation in a rat subcutaneous tissue
- Effect of MBCP block as carrier of rhBMP-2 in combination with ePTFE membrane on bone formation in rat calvarial defects
- Comparative analysis of carrier systems for delivering bone morphogenetic proteins
- Improvement of the osteogenic potential of ErhBMP-2-/EGCG-coated biphasic calcium phosphate bone substitute: in vitro and in vivo activity