J Periodontal Implant Sci.
2019 Apr;49(2):114-126. 10.5051/jpis.2019.49.2.114.
Improvement of the osteogenic potential of ErhBMP-2-/EGCG-coated biphasic calcium phosphate bone substitute: in vitro and in vivo activity
- Affiliations
-
- 1Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. kst72@snu.ac.kr
- 2Department of Dental Biomaterials, Institute of Biomaterials-Implant, Wonkwang University School of Dentistry, Iksan, Korea.
- KMID: 2444223
- DOI: http://doi.org/10.5051/jpis.2019.49.2.114
Abstract
- PURPOSE
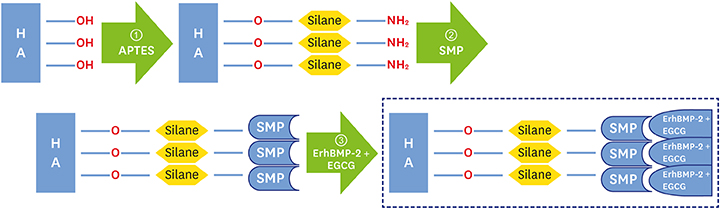
The aim of this study was to evaluate the enhancement of osteogenic potential of biphasic calcium phosphate (BCP) bone substitute coated with Escherichia coli-derived recombinant human bone morphogenetic protein-2 (ErhBMP-2) and epigallocatechin-3-gallate (EGCG).
METHODS
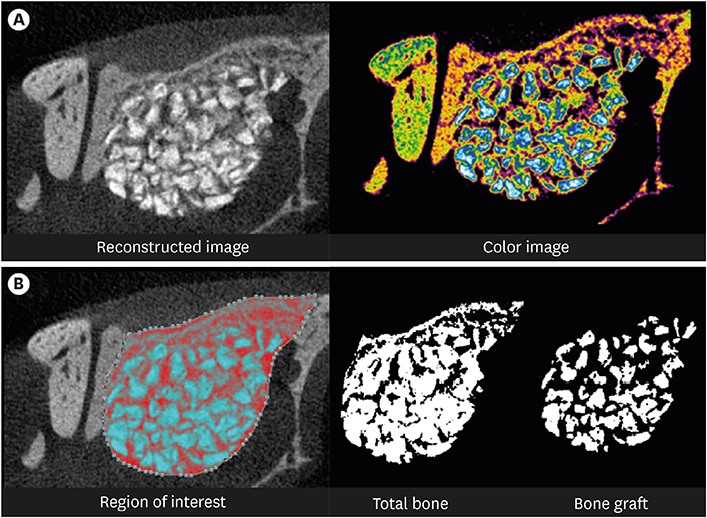
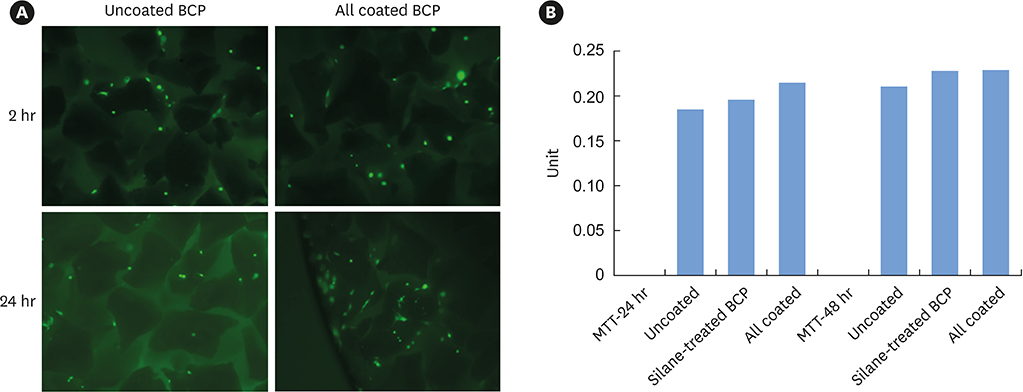
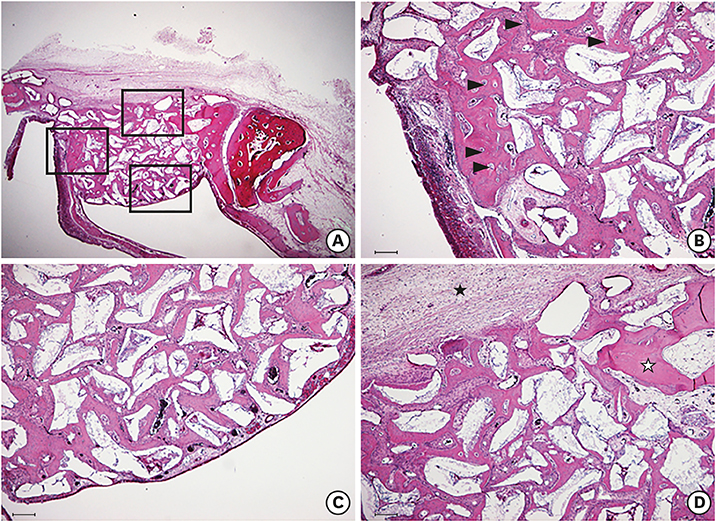
The cell viability, differentiation, and mineralization of osteoblasts was tested with ErhBMP-2-/EGCG solution. Coated BCP surfaces were also investigated. Standardized, 6-mm diameter defects were created bilaterally on the maxillary sinus of 10 male New Zealand white rabbits. After removal of the bony windows and elevation of sinus membranes, ErhBMP-2-/EGCG-coated BCP was applied on one defect in the test group. BCP was applied on the other defect to form the control group. The animals were sacrificed at 4 or 8 weeks after surgery. Histologic and histometric analyses of the augmented graft and surrounding tissue were performed.
RESULTS
The 4-week and 8-week test groups showed more new bone (%) than the corresponding control groups (P<0.05). The 8-week test group showed more new bone (%) than the 4-week test group (P<0.05).
CONCLUSIONS
ErhBMP-2-/EGCG-coated BCP was effective as a bone graft material, showing enhanced osteogenic potential and minimal side effects in a rabbit sinus augmentation model.
Keyword
MeSH Terms
Figure
Reference
-
1. Bessho K, Konishi Y, Kaihara S, Fujimura K, Okubo Y, Iizuka T. Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg. 2000; 38:645–649.
Article2. Lee JH, Kim CS, Choi KH, Jung UW, Yun JH, Choi SH, et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010; 31:3512–3519.
Article3. Long S, Truong L, Bennett K, Phillips A, Wong-Staal F, Ma H. Expression, purification, and renaturation of bone morphogenetic protein-2 from Escherichia coli . Protein Expr Purif. 2006; 46:374–378.
Article4. Vallejo LF, Brokelmann M, Marten S, Trappe S, Cabrera-Crespo J, Hoffmann A, et al. Renaturation and purification of bone morphogenetic protein-2 produced as inclusion bodies in high-cell-density cultures of recombinant Escherichia coli. J Biotechnol. 2002; 94:185–194.
Article5. Choi KH, Moon K, Kim SH, Yun JH, Jang KL, Cho KS. Purification and biological activity of recombinant human bone morphogenetic protein-2 produced by E. coli expression system. J Korean Acad Periodontol. 2008; 38:41–50.
Article6. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008; 8:1011–1018.
Article7. Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000; 27:479–486.
Article8. Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine(Phila Pa 1976). 2006; 31:2813–2819.
Article9. Choi H, Park NJ, Jamiyandorj O, Choi KH, Hong MH, Oh S, et al. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with two concentrations of expressed recombinant human bone morphogenetic protein 2. J Periodontal Implant Sci. 2012; 42:119–126.
Article10. Yang CS, Landau JM. Effects of tea consumption on nutrition and health. J Nutr. 2000; 130:2409–2412.
Article11. Tosetti F, Noonan DM, Albini A. Metabolic regulation and redox activity as mechanisms for angioprevention by dietary phytochemicals. Int J Cancer. 2009; 125:1997–2003.
Article12. Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006; 72:1439–1452.
Article13. Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, et al. Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun. 2002; 292:94–101.
Article14. Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004; 39:300–307.
Article15. Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem. 2007; 18:341–347.
Article16. Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Yamauchi J, Harada A, et al. (-)-Epigallocatechin gallate inhibits basic fibroblast growth factor-stimulated interleukin-6 synthesis in osteoblasts. Horm Metab Res. 2008; 40:674–678.
Article17. Tokuda H, Takai S, Matsushima-Nishiwaki R, Akamatsu S, Hanai Y, Hosoi T, et al. (--)-Epigallocatechin gallate enhances prostaglandin F2alpha-induced VEGF synthesis via upregulating SAPK/JNK activation in osteoblasts. J Cell Biochem. 2007; 100:1146–1153.
Article18. Kato K, Otsuka T, Adachi S, Matsushima-Nishiwaki R, Natsume H, Kozawa O, et al. (-)-Epigallocatechin gallate inhibits thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Mol Med Rep. 2011; 4:297–300.
Article19. Kim S, Jung UW, Lee YK, Choi SH. Effects of biphasic calcium phosphate bone substitute on circumferential bone defects around dental implants in dogs. Int J Oral Maxillofac Implants. 2011; 26:265–273.20. Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003; 14:195–200.21. Choi H, Park NJ, Jamiyandorj O, Hong MH, Oh S, Park YB, et al. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with synthetic cell binding peptide sequences. J Periodontal Implant Sci. 2012; 42:166–172.
Article22. Shin YS, Seo JY, Oh SH, Kim JH, Kim ST, Park YB, et al. The effects of ErhBMP-2-/EGCG-coated BCP bone substitute on dehiscence around dental implants in dogs. Oral Dis. 2014; 20:281–287.
Article23. Xiao SJ, Textor M, Spencer ND. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir. 1998; 14:5507–5516.
Article24. Durrieu MC, Pallu S, Guillemot F, Bareille R, Amédée J, Baquey CH, et al. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J Mater Sci Mater Med. 2004; 15:779–786.
Article25. Choi Y, Yun JH, Kim CS, Choi SH, Chai JK, Jung UW. Sinus augmentation using absorbable collagen sponge loaded with Escherichia coli-expressed recombinant human bone morphogenetic protein 2 in a standardized rabbit sinus model: a radiographic and histologic analysis. Clin Oral Implants Res. 2012; 23:682–689.
Article26. Misch CE. Contemporary implant dentistry. St. Louis (MO): Mosby;1999.27. Yun JH, Kim CS, Cho KS, Chai JK, Kim CK, Choi SH. (-)-Epigallocatechin gallate induces apoptosis, via caspase activation, in osteoclasts differentiated from RAW 264.7 cells. J Periodontal Res. 2007; 42:212–218.
Article28. Xu H, Shimizu Y, Ooya K. Histomorphometric study of the stability of newly formed bone after elevation of the floor of the maxillary sinus. Br J Oral Maxillofac Surg. 2005; 43:493–499.
Article29. Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clin Oral Implants Res. 2002; 13:405–409.
Article30. Frenken JW, Bouwman WF, Bravenboer N, Zijderveld SA, Schulten EA, ten Bruggenkate CM. The use of Straumann Bone Ceramic in a maxillary sinus floor elevation procedure: a clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin Oral Implants Res. 2010; 21:201–208.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with two concentrations of expressed recombinant human bone morphogenetic protein 2
- Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with synthetic cell binding peptide sequences
- Four-week histologic evaluation of grafted calvarial defects with adjunctive hyperbaric oxygen therapy in rats
- Comparison of the Bone Union Rates Using a Local Autobone and Bone Graft Substitute Mixed Graft in Lumbar Posterolateral Fusion
- Effects of biphasic calcium phosphate on bone formation in human fetal osteoblasts