J Korean Med Sci.
2004 Dec;19(6):853-858. 10.3346/jkms.2004.19.6.853.
Differential Expression of TGF-beta Isoforms During Differentiation of HaCaT Human Keratinocyte Cells: Implication for the Separate Role in Epidermal Differentiation
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Kyung Hee University, Seoul, Korea. nikim@khmc.or.kr
- 2East-West Medical Research Institute, College of Medicine, Kyung Hee University, Seoul, Korea.
- KMID: 1778568
- DOI: http://doi.org/10.3346/jkms.2004.19.6.853
Abstract
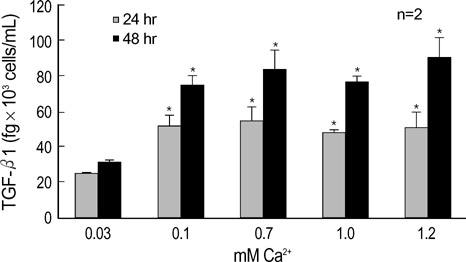
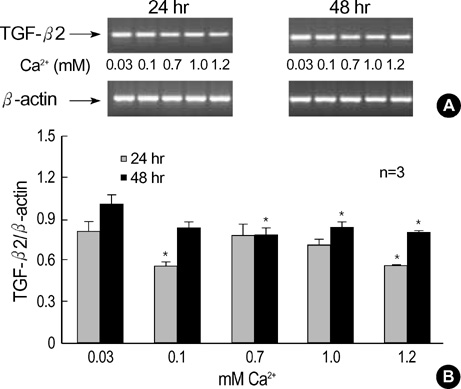
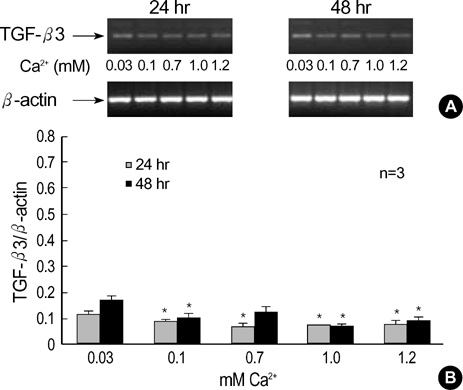
- The three mammalian isoforms of transforming growth factor-beta (TGF-beta1, beta2, beta3) are potent regulators of cell growth, differentiation, and extracellular matrix deposition. To study their role in skin differentiation, we investigated the expression of TGF-beta isoforms on cell growth and differentiation induction of the human keratinocyte cell line, HaCaT by elevating the Ca2+ concentration. An ELISA and RT-PCR assay revealed secreted TGF-beta 1 protein and TGF-beta1 mRNA were increased during calci-um-induced differentiation. In contrast, major differences were seen for TGF-beta 2 and TGF-beta 3 mRNA which were decreased during differentiation, but TGF-beta 2 and TGF-3beta protein were not evident on an ELISA. These results suggest different functions for each TGF-beta isoforms in epidermal differentiation, such that TGF-beta 1 is associ-ated with the more differentiated state, and TGF-beta 2 and TGF-beta 3 may be associ-ated the more proliferated state.
MeSH Terms
Figure
Cited by 1 articles
-
Differential Expression of TGF-β Isoforms in Human Kerationocytes by Narrow Band UVB
Moon Chul Jung, Min Kyung Shin, Kyung Kook Hong, Ki Heon Jeong, Nack In Kim
Ann Dermatol. 2008;20(3):113-119. doi: 10.5021/ad.2008.20.3.113.
Reference
-
1. Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993. 73:713–724.
Article2. Hennings H, Holbrook KA. Calcium regulation of cell-cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp Cell Res. 1983. 143:127–142.3. Ito Y, Sarkar P, Mi Q, Wu N, Bringas P Jr, Liu Y, Reddy S, Maxson R, Deng C, Chai Y. Overexpression of Smad2 reveals its concerted action with Smad4 in regulating TGF-beta-mediated epidermal homeostasis. Dev Biol. 2001. 236:181–194.4. Massague J. Receptors for the TGF-β family. Cell. 1992. 69:1067–1070.
Article5. Kingsley D. The TGF-β superfamily; new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994. 8:133–146.6. Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF-β1, TGF-β2, and TGF-β3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991. 115:1091–1105.7. Levine JH, Moses HL, Gold LI, Nanney LB. Spatial and temporal patterns of immunoreactive TGF-β1, β2, and β3 during excisional wound repair. Am J Pathol. 1993. 143:368–380.8. Gold LI, Saxena B, Mittal KR, Marmor M, Goswami S, Nactigal L, Korc M, Demopoulos RI. Increased expression of transforming growth factor-β isoforms and basic fibroblast growth factor in complex hyperplasia and adenocarcinoma of the endometrium: evidence for paracrine and autocrine action. Cancer Res. 1994. 54:2347–2358.9. Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. Immunohistochemical staining for transforming growth factor-β1 associates with disease progression in human breast cancer. Cancer Res. 1992. 52:6949–6952.10. Gold LI, Jussila T, Fusenig NE, Stenback F. TGF-β isoforms are differentially expressed in increasing malignant grades of HaCaT keratinocytes, suggesting separate roles in skin carcinogenesis. J Pathol. 2000. 190:579–588.
Article11. Missero C, Ramon Y, Cajal S, Dotto GP. Escape from transforming growth factor-β control and oncogene cooperation in skin tumor development. Proc Natl Acad Sci USA. 1991. 88:9613–9617.12. Watt FM, Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982. 295:434–436.
Article13. Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989. 109:1207–1217.
Article14. Wataya-Kaneda M, Hashimoto K, Kato M, Miyazono K, Yoshikawa K. Differential localization of TGF-beta-precursor isotypes in psoriatic human skin. J Dermatol Sci. 1996. 11:183–188.15. Breitkreutz D, Schoop VM, Mirancea N, Baur M, Stark HJ, Fusenig NE. Epidermal differentiation and basement membrane formation by HaCaT cells in surface transplants. Eur J Cell Biol. 1998. 75:273–286.
Article16. Schmid P, Cox D, Bilbe G, McMaster G, Morrison C, Stahelin H, Luscher N, Seiler W. TGF-betas and TGF-beta type II receptor in human epidermis: differential expression in acute and chronic skin wounds. J Pathol. 1993. 171:191–197.17. Edmondson SR, Murashita MM, Russo VC, Wraight CJ, Werther GA. Expression of insulin-like growth factor binding protein-3 (IGFBP-3) in human keratinocytes is regulated by EGF and TGFbeta1. J Cell Physiol. 1999. 179:201–207.18. Edmondsion SR, Werther GA, Wraight CJ. Calcium regulates the expression of insulin-like growth factor binding protein-3 by the human keratinocyte cell line HaCaT. J Invest Dermatol. 2001. 116:491–497.19. Glick AB, Danielpour D, Morgan D, Sporn MB, Yuspa SH. Induction and autocrine receptor binding of transforming growth factor-β2 during terminal differentiation of primary mouse keratinocytes. Mol Endocrinol. 1990. 4:46–52.
Article20. Matsumoto K, Hashimoto K, Hashiro M, Yoshimasa H, Yoshikawa K. Modulation of growth and differentiation in normal human keratinocytes by transforming growth factor-beta. J Cell Physiol. 1990. 145:95–101.21. Kato M, Ishizaki A, Hellman U, Wernstedt C, Kyogoku M, Miyazono K, Heldin CH, Funa K. A human keratinocyte cell line produces two autocrine growth inhibitors, transforming growth factor-β and insulin-like growth factor binding protein-6, in a calcium- and cell density-dependent manner. J Biol Chem. 1995. 270:12373–12379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Separation of Human Epidermal Cells by Using Percoll
- Differential Expression of TGF-beta Isoforms in Human Kerationocytes by Narrow Band UVB
- Single Low-Dose Radiation Induced Regulation of Keratinocyte Differentiation in Calcium-Induced HaCaT Cells
- Organotypic Culture of HaCaT cells: Use of Dermal Substrate that Combines de-epidermized Dermis with Fibroblast-populated Collagen Matrix
- Immunohistochemical Study on Expression of Transforming Growth Factor-betas in Keratoacanthoma and Squamous Cell Carcinoma