Ann Dermatol.
2008 Sep;20(3):113-119. 10.5021/ad.2008.20.3.113.
Differential Expression of TGF-beta Isoforms in Human Kerationocytes by Narrow Band UVB
- Affiliations
-
- 1Department of Dermatology, School of Medicine, Kyung Hee University, Seoul, Korea. nikim@khmc.or.kr
- KMID: 2156375
- DOI: http://doi.org/10.5021/ad.2008.20.3.113
Abstract
-
BACKGROUND: Transforming growth factor-beta (TGF-beta), a multifunctional growth factor, has three isoforms: TGF-beta1, TGF-beta2, and TGF-beta3. Different isoforms of TGF-beta are associated with different proliferation and differentiation states of the epidermis. Narrow band ultraviolet B (NBUVB) emits a concentrated UVB source of 311 nm. NBUVB 1,000 mJ/cm2 induces apoptosis in approximately 50% of keratinocytes.
OBJECTIVE
The purpose of this study was to evaluate whether irradiation with NBUVB would alter the expression and production of TGF-beta1, 2, and 3.
METHODS
We measured TGF-beta1, 2, and 3 mRNA and TGF-beta1 and 2 protein levels at 800, 1,000, and 1,200 mJ/cm2 for 24 hours and 48 hours.
RESULTS
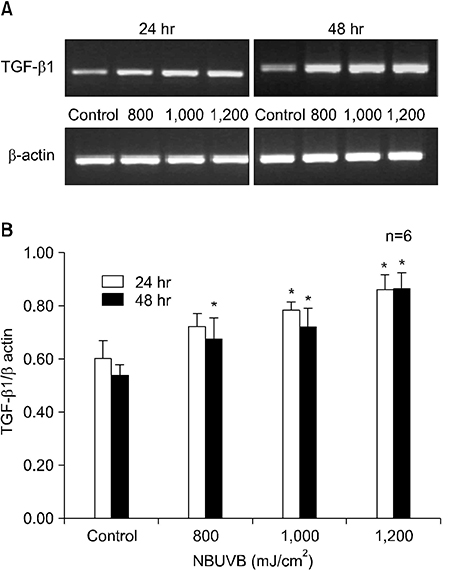
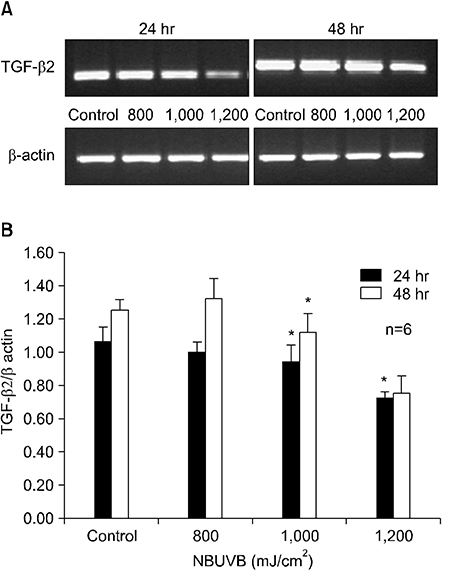
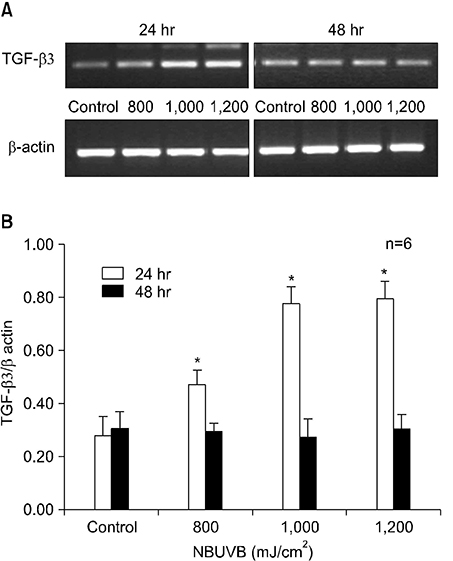
TGF-beta1 mRNA levels were increased at both 24 hr and 48 hr, TGF-beta2 mRNA levels were decreased at both 24 hr and 48 hr, and TGF-beta3 mRNA levels were increased at 24 hr and similar to control at 48 hr. TGF-beta1 protein levels were increased at 48 hr but decreased at 24 hr. TGF-beta2 protein levels were decreased at both 24 hr and 48 hr.
CONCLUSION
The results suggest a possible role for TGF-beta1 after NBUVB irradiation and opposing roles for TGF-beta1 and TGF-beta2 isoforms in NBUVB irradiation.
MeSH Terms
-
Apoptosis
Enzyme Multiplied Immunoassay Technique
Epidermis
Humans
Keratinocytes
Protein Isoforms
RNA, Messenger
Transforming Growth Factor beta
Transforming Growth Factor beta1
Transforming Growth Factor beta2
Transforming Growth Factor beta3
Protein Isoforms
RNA, Messenger
Transforming Growth Factor beta
Transforming Growth Factor beta1
Transforming Growth Factor beta2
Transforming Growth Factor beta3
Figure
Reference
-
1. Griffiths CE, Clark CM, Chalmers RJ, Li Wan Po A, Williams HC. A systemic review of treatments for severe psoriasis. Health Technology Assessment. 2000; 4:1–125.2. Chaturvedi V, Qin JZ, Denning MF, Choubey D, Diaz MO, Nickoloff BJ. Apoptosis in proliferating, senescent and immortalized keratinocytes. J Biol Chem. 1999; 274:23358–23367.
Article3. Cotton J, Spandau DF. Ultraviolet B-radiation dose influences the induction of apoptosis and p53 in human keratinocytes. Radiat Res. 1997; 147:148–155.
Article4. Petit-Frere C, Capulas F, Lyon DA, Norbury CJ, Lowe JE, Clingen PH, et al. Apoptosis and cytokine release induced by ionizing or ultraviolet B radiation in primary and immortalized human keratinocytes. Carcinogenesis. 2000; 21:1087–1095.
Article5. Aufiero BM, Talwar H, Young C, Krishnan M, Hatfield JS, Lee HK, et al. Narrow-band UVB induces apoptosis in human keratinocytes. J Photochem Photobiol B. 2006; 82:132–139.
Article6. Gandarillis A. Epidermal differentiation, apoptosis and senescence: common pathways. Exp Gerontol. 2000; 35:53–62.
Article7. Seitz C, Freiberg R, Hinata K, Khavari P. NFkappaB determines localization and features of cell death in epidermis. J Clin Invest. 2000; 105:253–260.8. Petrocelli T, Poon R, Drucker DJ, Slingerland JM, Rosen CF. UVB radiation induces p21Cip/WAF1 and mediates Gl and S phase cheekpoints. Oncogene. 1996; 12:1387–1396.9. Van Ruissen F, van Erp PE, de Jongh GJ, Boezeman JB, van de Kerkhof PC, Schalkwijk J. Cell kinetic characterization of growth arrest in cultured human keratinocytes. J Cell Sci. 1994; 107:2219–2228.
Article10. Brenner M, Degitz K, Besch R, Berking C. Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Br J Dermatol. 2005; 153:733–739.
Article11. Massague J. Receptors for the TGF-β family. Cell. 1992; 69:1067–1070.
Article12. Ignolz RA, Massague J. Transforming growth factor-β stimulatesthe expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986; 261:4337–4345.13. Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type 1collagen mRNA levels by transforming grow factor-beta. J Biol Chem. 1987; 262:6443–6446.
Article14. Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, et al. Tranforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986; 83:4167–4171.
Article15. Min BW, Woo KM, Lee G, Park NH. Terminal differentiation of normal human oral keratinocytes is associated with enhanced cellular TGF-beta and phospholipase C-gamma 1 levels and apoptotic cell death. Exp Cell Res. 1999; 249:377–385.
Article16. Merryman JI, Neilsen N, Stanton DD. Transforming growth factor-beta enhances the ultravioletmediated stress response in p53-/-keratinocytes. Int J Oncol. 1998; 13:781–789.17. Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF-β1, TGF-β2, and TGF-β3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991; 115:1091–1105.
Article18. Levine JH, Moses HL, Gold LI, Nanney LB. Spatial and temporal patterns of immunoreactive TGF-β 1, 2, and 3 during excisional wound repair. Am J Pathol. 1993; 143:368–380.19. Gold LI, Saxena B, Mittal KR, Marmor M, Goswami S, Nactial L, et al. Increased expression of transforming growth factor-β isoforms and basic fibroblast growth factor in complex hyperplasia and adenocarcinoma of the endometrium: evidence for paracrine and autocrine action. Cancer Res. 1994; 54:2347–2358.20. Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. Immunohistochemical staining for transforming growth factor-β1 associates with disease progression in human breast cancer. Cancer Res. 1992; 52:6949–6952.21. Gold LI, Jussila T, Fusenig NE, Stenback F. TGF-β isoforms are differentially expressed in increasing malignant grades of HaCaT keratinocytes, suggesting separate roles in skin carcinogenesis. J Pathol. 2000; 190:579–588.
Article22. Cho HR, Hong SB, Kim YI, Lee JW, Kim NI. Differential expression of TGF-beta isoforms during differentiation of HaCaT human keratinocyte cells: implication for the separate role in epidermal differentiation. J Korean Med Sci. 2004; 19:853–858.
Article23. Flisiak I, Chodynicka B, Porebski P, Flisiak R. Association between psoriasis severity and transforming growth factor β1 and β2 in plasma and scales form psoriatic lesion. Cytokine. 2002; 19:121–125.
Article24. Flisiak I, Porebski P, Flisiak R, Chondynicka B. Plasma transforming growth factor β1 as a biomarker of psoriasis activity and treatment efficacy. Biomarkers. 2003; 8:437–443.
Article25. Watt FM, Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982; 295:434–436.
Article26. Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006; 47:1886–1894.
Article27. Yu L, Border WA, Huang Y, Noble NA. TGF-beta isoforms in renal fibrogenesis. Kidney Int. 2003; 64:844–856.28. Gorvy DA, Herrick SE, Shah M, Ferguson MW. Experimental manipulation of transforming growth factor-beta isoforms significantly affects adhesion formation in a murine surgical model. Am J Pathol. 2005; 167:1005–1019.
Article29. Matejuk A, Dwyer J, Hopke C, Vandenbark AA, Offner H. Opposing roles for TGF-β1 and TGF-β3 isoforms in experimental autoimmune encephalomyelitis. Cytokine. 2004; 25:45–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Expression of TGF-beta Isoforms During Differentiation of HaCaT Human Keratinocyte Cells: Implication for the Separate Role in Epidermal Differentiation
- Narrow-Band UVB Phototherapy in Korean Psoriasis Patients
- Efficacy of Narrow-Band UVB Phototherapy in Vitiligo Patients
- The Study of Dose Incremental Regimen for Narrow-Band UVB Phototherapy in Psoriatic patients
- Contrasting Roles of Different Endoglin Forms in Atherosclerosis