J Korean Med Sci.
2006 Jun;21(3):445-451. 10.3346/jkms.2006.21.3.445.
Effects of alpha-Tocopherol on Cadmium-Induced Toxicity in Rat Testis and Spermatogenesis
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Dongguk University, Gyeongju, Korea. y31354@hotmail.com
- 2Department of Pathology, College of Medicine, Dongguk University, Gyeongju, Korea.
- KMID: 1778426
- DOI: http://doi.org/10.3346/jkms.2006.21.3.445
Abstract
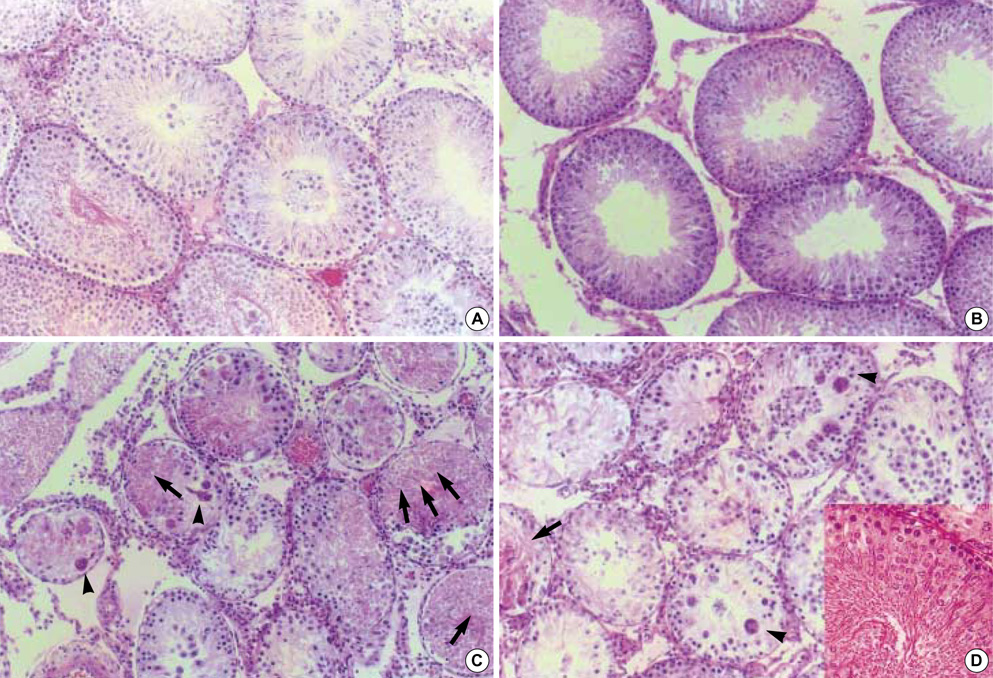
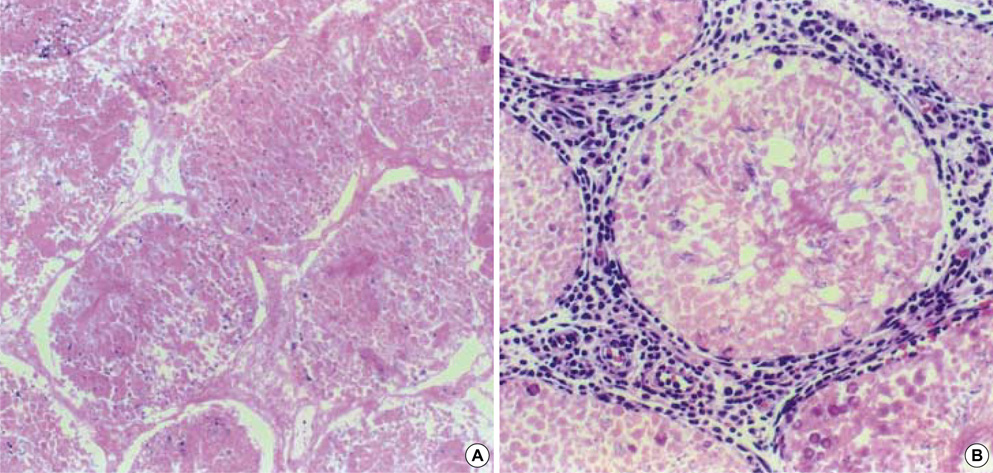
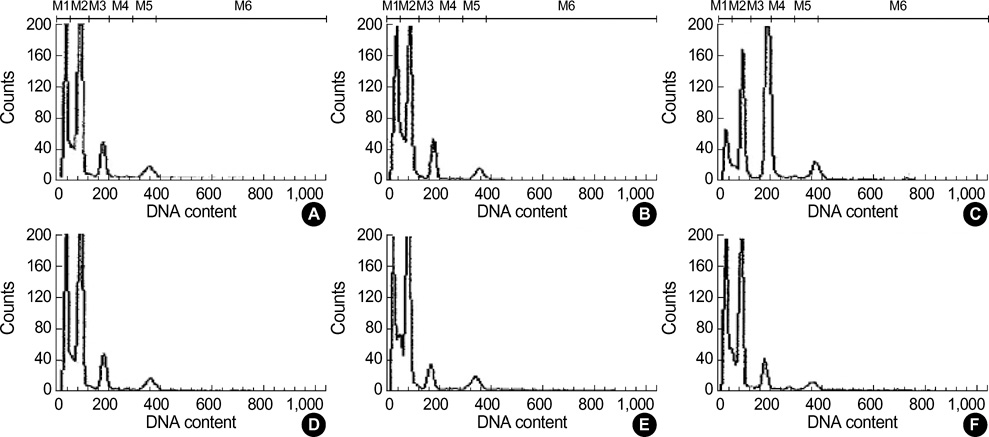
- Cadmium is known to exert toxic effects on multiple organs, including the testes. To determine if alpha-tocopherol, an antioxidant, could protect testicular tissues and spermatogenesis from the toxic effects of cadmium, six-week old male Sprague-Dawley rats were randomized to receive cadmium at doses of 0 (control), 1, 2, 4 or 8 mg/kg by the intraperitoneal route (Group A) or alpha-tocopherol for 5 days before being challenged with cadmium (Group B) in an identical dose-dependent manner. When both groups received cadmium at 1 mg/kg, there were no changes in testicular histology relative to controls. When Group A received cadmium at 2 mg/kg, undifferentiated spermatids and dead Sertoli cells increased in the seminiferous tubules while interstitial cells decreased and inflammatory cells increased in the interstitial tissues. On flow cytometric analysis, the numbers of elongated spermatids (M1) and round spermatids (M2) decreased while 2c stage cells (M3, diploid) increased. In contrast, when Group B received cadmium at 2 mg/kg, the histological insults were reduced and the distribution of the germ cell population remained comparable to controls. However, alpha-tocopherol had no protective effects with higher cadmium doses of 4 and 8 mg/kg. These findings indicate that alpha-tocopherol treatment can protect testicular tissue and preserve spermatogenesis from the detrimental effects of cadmium but its effectiveness is dependent on the dose of cadmium exposed.
Keyword
MeSH Terms
Figure
Reference
-
1. Page AL, Chang AC. Foulkes EC, editor. Cadmium in the environment and its entry into terrestrial food chain crops. Handbook of Experimental Pharmacology. 1986. Berlin: Springer-Verlag;33–74.
Article2. Itokawa Y, Abe T, Tanaka S. Bone changes in experimental chronic cadmium poisoning: radiological and biological approaches. Arch Environ Health. 1973. 26:241–244.3. Din WS, Frazier JM. Protective effect of metallothionein on cadmium toxicity in isolated rat hepatocytes. Biochem J. 1985. 230:395–402.
Article4. Axelsson B, Piscator M. Renal damage after prolonged exposure to cadmium. An experimental study. Arch Environ Health. 1966. 12:360–373.5. Verschoor M. Renal function of workers with low-level cadmium exposure. Scand J Work Environ Health. 1987. 13:232–238.
Article6. Wong KL, Klaassen CD. Neurotoxic effects of cadmium in young rats. Toxicol Appl Pharmacol. 1982. 63:330–337.7. Parizek J. The effect of a subcutaneous injection of cadmium salts on the ovaries of adult rats in persistent oestrus. J Reprod Fertil. 1968. 17:559–562.
Article8. Benoff S, Jacob A, Hurley IR. Male infertility and environmental exposure to lead and cadmium. Human Reproduction Update. 2000. 6:107–121.
Article9. Koizumi T, Li ZG. Role of oxidative stress in single-dose, cadmium-induced testicular cancer. J Toxicol Environ Health. 1992. 37:25–36.
Article10. Jamall IS, Smith JC. Effects of cadmium on glutathione peroxidase, superoxide dismutase, and lipid peroxidation in the rat heart: a possible mechanism of cadmium cardiotoxicity. Toxicol Appl Pharmacol. 1985. 80:33–42.
Article11. Manca D. In vitro and in vivo responses of rat tissues to cadmium-induced cadmiuminduced lipid peroxidation. Bull Environ Contam Toxicol. 1991. 46:929–936.12. Hussain T, Shukla GS, Chandra SV. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacol Toxicol. 1987. 60:355–358.13. Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. J Androl. 1995. 16:464–481.14. Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987. 8:338–348.
Article15. Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989. 41:183–197.16. Palamanda JR, Kehrer JP. Involvement of vitamin E and protein thiols in the inhibition of microsomal lipid peroxidation by glutathione. Lipids. 1993. 28:427–431.
Article17. Ernster L, Forsmark P, Nordenbrand K. The mode of action of lipid-soluble antioxidants in biological membranes: relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. Biofactors. 1992. 3:241–248.
Article18. Zante J, Schuman J, Gohde W, Hacker U. DNA-fluorometry of mammalian sperm. Histochemistry. 1977. 54:1–7.
Article19. Sharpe RM. Knobil E, Neill JD, editors. Regulation of spermatogenesis. The Physiology of Reproduction. 1994. New York: Raven Press;1363–1434.20. Clermont Y. Quantitative analysis of spermatogenesis of the rat: a revised model for the renewal of spermatogonia. Am J Androl. 1962. 2:37–58.
Article21. Roosen-Runge EC. Germinal cell loss in normal metazoan spermatogenesis. J Reprod fertil. 1973. 35:339–348.22. De Rooij G, Lok D. Regulation of the density of spermatogonia in the seminiferous epithelium of the Chinese hamster: II. Differentiating spermatogonia. Anat Rec. 1987. 217:131–136.
Article23. Russell LD, Alger LE, Nequin LG. Hormonal control of pubertal spermatogenesis. Endocrinology. 1987. 120:1615–1632.
Article24. Richburg JH, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol Appl Pharmacol. 1996. 137:42–50.
Article25. Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991. 113:689–699.
Article26. Miraglia SM, Hayashi H. Histomorphometry of immature rat testis after heating. J Morphol. 1993. 217:65–74.
Article27. Kwon KS, Cho KJ, Yang EJ. The study of anatomical and biochemical effects of cadmium on the testis in rats. Dev Reprod. 1997. 1:125–132. [in Korean].28. Srinivas M, Agarwala S, Gupta SD, Das SN, Jha P, Misro MM, Mitra DK. Effect if cyclosporine on fertility in male rats. Pediatr Surg Int. 1998. 13:388–391.29. Hacker U, Schumann J, Gohde W. Effects of acute gamma-irradiation on spermatogenesis as revealed by flow cytometry. Acta Radiol Oncol. 1980. 19:361–368.
Article30. Suleiman SA, Elamin AM, Zaki ZM, El-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996. 17:530–537.31. Therond P, Auger J, Legrand A, Jouannet P. Alpha-tocopherol in human spermatozoa and seminal plasma: relationships with motility, antioxidant enzymes and leukocytes. Mol Hum Reprod. 1996. 2:739–744.32. Askari HA, Check JH, Peymer N, Bollendrof A. Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Arch Androl. 1994. 33:11–15.
Article33. Ollero M, Blanco TM, Lopez-Perez MJ, Cebrian Perez JA. Surface changes associated with ram sperm cryopreservation revealed by counter-current distribution in an aqueous two-phase system. Effect of different cryoprotectants. J Chromatogr B Biomed Appl. 1996. 680:157–164.
Article34. Beconi MT, Francia CR, Mora NG, Affranchino MA. Influence of antioxidants on SOD activity in bovine sperm. Biochem Int. 1991. 23:545–553.35. O'Flaherty C, Beconi M, Beorlegui N. Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia. 1997. 29:269–275.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction Of Metallothionein And Toxicity In Acute Cadmium Intoxicated Rat
- Protective Effect of Several Metals Against Cadmium Injury to Mouse Testicle
- Vitamin E: alpha-Tocopherol and the Other Forms of Vitamin E
- Effect of Cadmium Chloride on the Mature Rat Testis
- Effects of testicular biopsy in rat testis: comparison of open surgical percutaneous needle and biopsy gun biopsy technique