J Korean Med Sci.
2009 Jan;24(Suppl 1):S195-S203. 10.3346/jkms.2009.24.S1.S195.
Aldosterone-induced TGF-beta1 Expression is Regulated by Mitogen-Activated Protein Kinases and Activator Protein-1 in Mesangial Cells
- Affiliations
-
- 1Renal Research Laboratory, Department of Internal Medicine, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- 2Division of Nephrology, Department of Internal Medicine, The Catholic University of Korea, College of Medicine, Seoul, Korea. kimcmc@catholic.ac.kr
- KMID: 1778162
- DOI: http://doi.org/10.3346/jkms.2009.24.S1.S195
Abstract
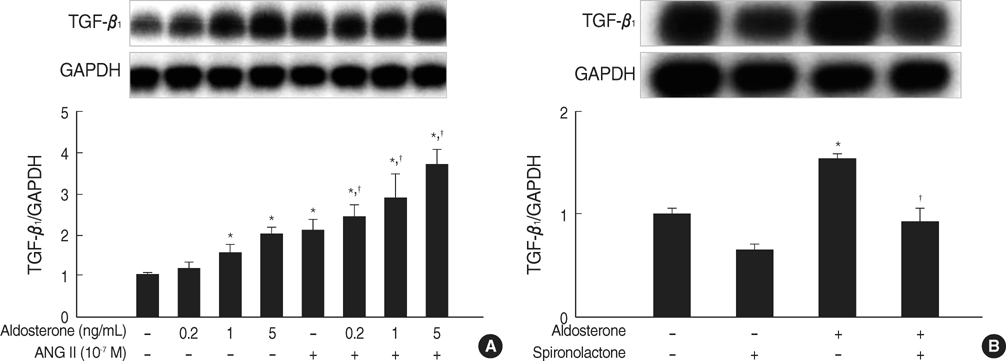
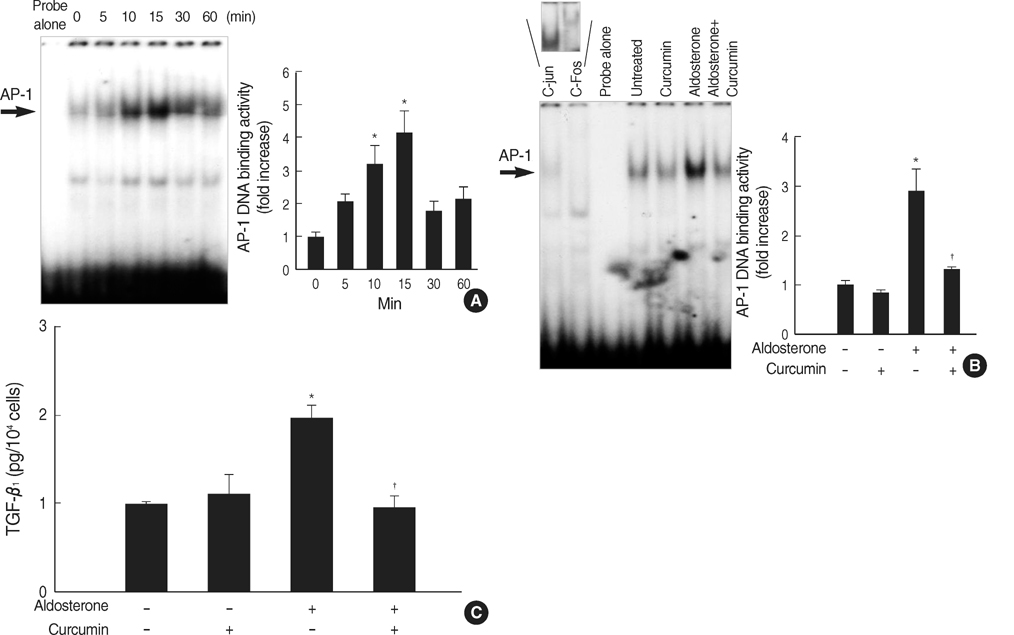
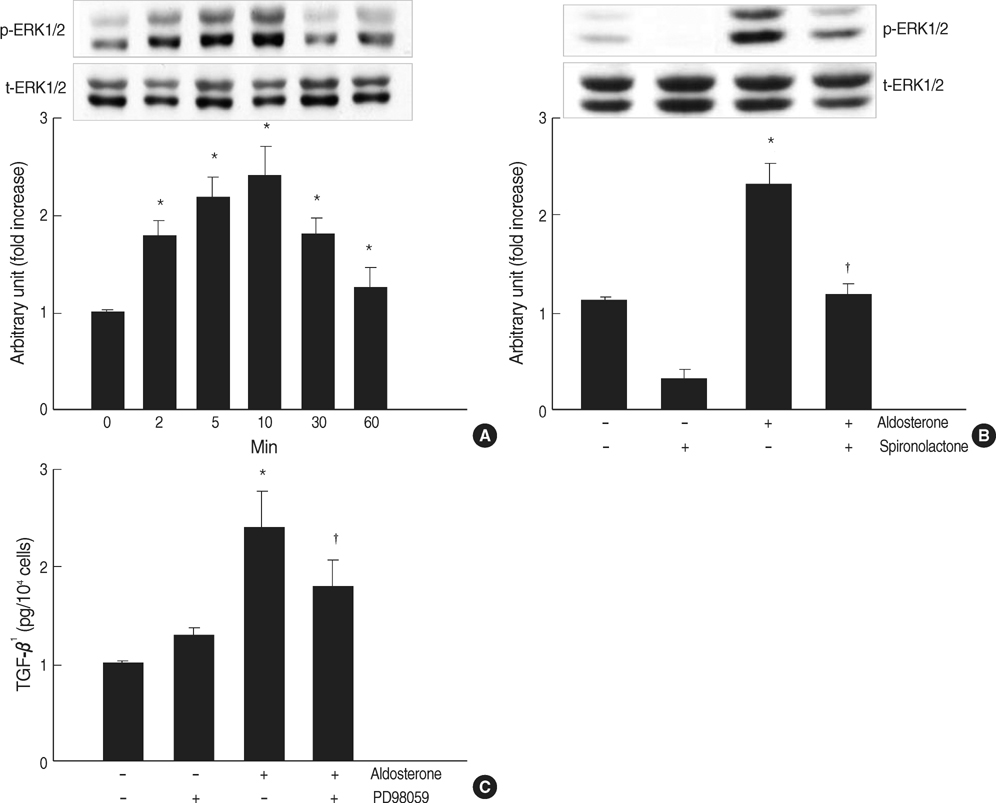
- Aldosterone has been shown to stimulate renal TGF-beta1 expression. However, the mechanisms for aldosterone-induced TGF-beta1 expression have not been clearly determined in mesangial cells. We examined the role of extracellular-signal regulated kinase 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK) and activator protein- 1 (AP-1) in the aldosterone-induced TGF-beta1 expression in rat mesangial cells. TGF-beta1 protein in the conditioned medium released from rat mesangial cells was measured by sandwich ELISA, TGF-beta1 mRNA expression was analyzed by Northern blotting, AP-1 DNA binding activity was measured by EMSA and the ERK1/2, JNK activity was analyzed by western blotting. Aldosterone significantly stimulated TGF-beta1 protein production and TGF-beta1 mRNA expression in mesangial cells in a dose-dependent manner. Aldosterone significantly increased AP-1 DNA binding activity in mesangial cells. Pre-treatment of cells with AP-1 inhibitor, curcumin, blocked aldosterone-induced AP-1 DNA binding activity as well as aldosterone-induced TGF-beta1 production. Aldosterone increased phosphorylation of ERK1/2 and JNK in mesangial cells. Pre-treatment of cells with ERK1/2 inhibitor, PD98059, or JNK inhibitor, SP600125 significantly inhibited aldosterone-induced ERK1/2 and JNK activity and subsequently TGF-beta1 production, respectively. We conclude that aldosteroneinduced TGF-beta1 expression in mesangial cells is regulated by the ERK1/ 2, JNK and AP-1 intracellular signaling pathways.
Keyword
MeSH Terms
-
Aldosterone/*pharmacology
Animals
Culture Media, Conditioned/pharmacology
DNA/metabolism
Extracellular Signal-Regulated MAP Kinases/metabolism
*Gene Expression Regulation, Enzymologic
Humans
*MAP Kinase Signaling System
Mesangial Cells/*metabolism
Models, Biological
Phosphorylation
Protein Binding
Rats
Transcription Factor AP-1/*metabolism
Transforming Growth Factor beta1/*biosynthesis
Figure
Reference
-
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005. 99:S57–S65.
Article2. Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006. 17:2985–2991.3. Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996. 98:1063–1068.
Article4. Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB. Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol. 2005. 16:3306–3314.
Article5. Rocha R, Chander PN, Zuckerman A, Stier CT Jr. Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension. 1999. 33:232–237.
Article6. Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003. 63:1791–1800.
Article7. Feria I, Pichardo I, Juárez P, Ramírez V, González MA, Uribe N, García-Torres R, Lopez-Casillas F, Gamba G, Bobadilla NA. Therapeutic benefit of spironolactone in experimental chronic cyclosporine A nephrotoxicity. Kidney Int. 2003. 63:43–52.
Article8. Perez-Rojas J, Blanco JA, Cruz C, Trujillo J, Vaidya VS, Uribe N, Bonventre JV, Gamba G, Bobadilla NA. Mineralocorticoid receptor blockade confers renoprotection in preexisting chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol. 2007. 292:F131–F139.9. Fujisawa G, Okada K, Muto S, Fujita N, Itabashi N, Kusano E, Ishibashi S. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int. 2004. 66:1493–1502.
Article10. Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, Han KH, Kim HK, Kang YS, Han JY, Kim YS, Cha DR. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006. 17:1362–1372.
Article11. Juknevicius I, Segal Y, Kren S, Lee R, Hostetter TH. Effect of aldosterone on renal transforming growth factor-beta. Am J Physiol Renal Physiol. 2004. 286:F1059–F1062.12. Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006. 70:2116–2123.
Article13. Sato A, Hayashi K, Saruta T. Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens. 2005. 18:44–49.
Article14. Kreisberg JI, Karnovsky MJ. Glomerular cells in culture. Kidney Int. 1983. 23:439–447.
Article15. Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J Clin Invest. 1994. 93:2431–2437.16. Wu Z, Zhou Q, Lan Y, Wang Y, Xu X, Jin H. AP-1 complexes mediate oxidized LDL-induced overproduction of TGF-beta(1) in rat mesangial cells. Cell Biochem Funct. 2004. 22:237–247.17. Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995. 270:16483–16486.
Article18. Ahn JD, Morishita R, Kaneda Y, Lee KU, Park JY, Jeon YJ, Song HS, Lee IK. Transcription factor decoy for AP-1 reduces mesangial cell proliferation and extracellular matrix production in vitro and in vivo. Gene therapy. 2004. 11:916–923.
Article19. Smeal T, Binetruy B, Mercola D, Grover-Bardwick A, Heidecker G, Rapp UR, Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992. 12:3507–3513.
Article20. Guan Z, Tetsuka T, Baier LD, Morrison AR. Interleukin-1 beta activates c-jun NH2-terminal kinase subgroup of mitogen-activated protein kinases in mesangial cells. Am J Physiol. 1996. 270:F634–F641.
Article21. Nagai Y, Miyata K, Sun GP, Rahman M, Kimura S, Miyatake A, Kiyomoto H, Kohno M, Abe Y, Yoshizumi M, Nishiyama A. Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension. 2005. 46:1039–1045.
Article22. Nishiyama A, Yao L, Fan Y, Kyaw M, Kataoka N, Hashimoto K, Nagai Y, Nakamura E, Yoshizumi M, Shokoji T, Kimura S, Kiyomoto H, Tsujioka K, Kohno M, Tamaki T, Kajiya F, Abe Y. Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005. 45:710–716.
Article23. Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res. 2005. 97:434–442.
Article24. Nakano S, Kobayashi N, Yoshida K, Ohno T, Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res. 2005. 28:925–936.
Article25. Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-β1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007. 72:45–52.
Article26. Otsuka M, Negishi Y, Aramaki Y. Involvement of phosphatidylinositol-3-kanase and ERK pathways in the production of TGF-β1 by macrophages treated with liposomes composed of phosphatidylserine. FEBS Letters. 2007. 581:325–330.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardamonin Suppresses TGF-beta1-Induced Epithelial Mesenchymal Transition via Restoring Protein Phosphatase 2A Expression
- Mitogen-activated Protein Kinases in the Development of Normal and Diseased Kidneys
- TGF-beta1-induced PINCH-1-ILK-alpha-parvin complex formation regulates mesangial cell proliferation and hypertrophy
- The Inhibitory Effect of siRNAs on The High Glucose-Induced Overexpression of TGF-beta1 in Mesangial Cells
- Mitogen-activated Protein Kinases in Inflammation