J Korean Med Sci.
2006 Jun;21(3):430-435. 10.3346/jkms.2006.21.3.430.
The Inhibitory Effect of siRNAs on The High Glucose-Induced Overexpression of TGF-beta1 in Mesangial Cells
- Affiliations
-
- 1Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
- 2Department of Nephrology, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Pathology, College of Medicine, Catholic University School of Medicine, Daegu, Korea. kkpark@cu.ac.kr
- KMID: 1778423
- DOI: http://doi.org/10.3346/jkms.2006.21.3.430
Abstract
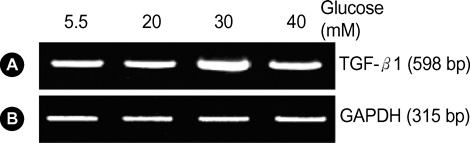
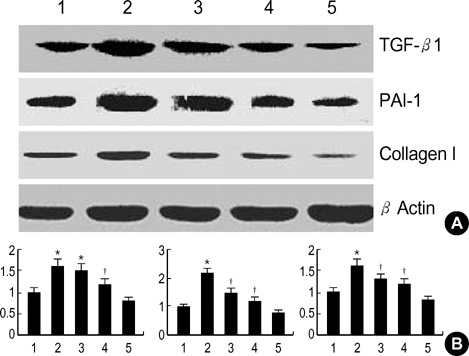
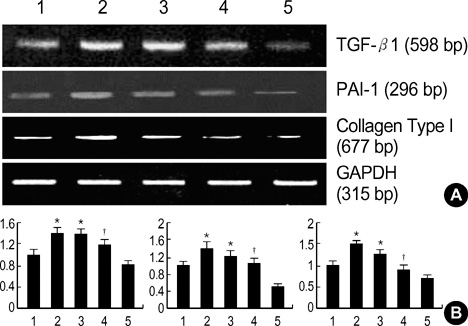
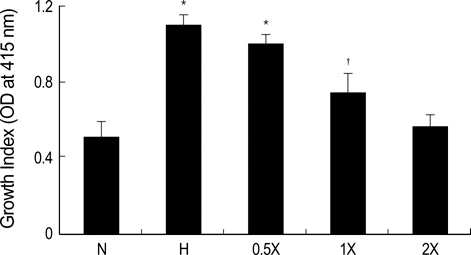
- Diabetic nephropathy is characterized by an expansion of the glomerular mesangium, caused by mesangial cell proliferation and an excessive accumulation of extracellar matrix (ECM) proteins, which eventually leading to glomerulosclerosis. TGF-beta1 was found to play an important role in the accumulation of ECM in the kidney. In this study, TGF-beta1 RNA interference was used as an effective therapeutic strategy. The inhibitory effect of TGF-beta1 small interfering RNAs (siRNAs) on the high glucose-induced overexpression of TGF-beta1 in rat mesangial ceys (RMCs). A high levels of glucose induces TGF-beta1 mRNA and protein, and TGF-beta1 siRNAs reduce the ability of high glucose to stimulate their expression. We also examined the inhibitory effect of TGF-beta1 siRNAs on the expression of plasminogen activator inhibitor (PAI)-1 and Collagen Type I which are down-regulators of TGF-beta1. The expression of TGF-beta1, PAI-1 and Collagen Type I was increased in RMCs that were stimulated by 30 mM glucose. TGF-beta1 siRNAs reduces high glucose-induced TGF-beta1, PAI-1, and Collagen Type I mRNA and protein expression in a dose-dependent manner. In conclusion, the present study demonstrates that TGF-beta1 siRNAs effectively inhibits TGF-beta1 mRNA and protein expression in RMCs. These suggest that TGF-beta1 siRNAs through RNAi may be a useful tool for developing new therapeutic applications for the treatment of diabetic nephropathy.
Keyword
MeSH Terms
-
Transforming Growth Factor beta1/*metabolism
Rats, Sprague-Dawley
Rats
RNA, Small Interfering/*metabolism
Microscopy, Fluorescence
Mesangial Cells/*metabolism
Male
Glucose/*metabolism
Glomerular Mesangium/*metabolism
Gene Expression Regulation
Diabetic Nephropathies/pathology
Collagen Type I/metabolism
Cells, Cultured
Cell Proliferation
Animals
Figure
Reference
-
1. Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 1999. 56:393–405.
Article2. Steffes MW, Bilous RW, Sutherland DE, Mauer SM. Cell and matrix components of the glomerular mesangium in type I diabetes. Diabetes. 1992. 41:679–684.
Article3. Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N Engl J Med. 1988. 318:1657–1666.
Article4. Jensen T. Pathogenesis of diabetic vascular disease; evidence for the role of reduced heparan sulfate proteoglycan. Diabetes. 1997. 2:98–100.
Article5. Grishok A, Mello CC. RNAi (Nematodes: Caenorhabditis elegans). Adv Genet. 2002. 46:339–360.6. Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994. 93:536–542.
Article7. Nahman NS Jr, Leonhart KL, Cosio FG, Herbert CL. Effects of high glucose on cellular proliferation and fibronectin production by cultured human mesangial cells. Kidney Int. 1992. 41:396–402.
Article8. Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest. 1997. 100:115–126.
Article9. Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN. High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-β1. Kidney Int. 1992. 42:647–656.10. Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J Clin Invest. 1994. 93:536–542.11. Hoffman BB, Sharma K, Zhu Y, Ziyadeh FN. Transcriptional activation of transforming growth factor-β1 in mesangial cell culture by high glucose concentration. Kidney Int. 1998. 54:1107–1116.
Article12. Zamore PD, Tuschl , Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000. 101:25–33.13. Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001. 409:363–366.
Article14. Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001. 293:1146–1150.
Article15. Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002. 26:199–213.
Article16. Leehey DJ, Song RH, Alari N, Singh AK. Decreased degradative enzymes in mesangial cells cultured in high glucose media. Diabetes. 1995. 44:929–935.
Article17. Kaname S, Uchida S, Ogata E, Kurokawa K. Autocrine secretion of transforming growth factor-β in cultured rat mesangial cells. Kidney Int. 1992. 42:1319–1327.
Article18. Chen S, Jim B, Ziyadeh FN. Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol. 2003. 6:532–543.19. Oh JH, Ha HJ, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-β1 and fibronectin synthesis. Kidney Int. 1998. 54:1872–1878.
Article20. Park IS, Kiyomoto H, Abboud SL, Abboud HE. Expression of transforming growth factor-β and type IV collagen in early streptozotocin-induced diabetes. Diabetes. 1997. 46:473–480.
Article21. Hill C, Flyvbjerg A, Gronbaek H, Petrik J, Hill DJ, Thomas CR, Sheppard MC, Logan A. The renal expression of transforming growth factor-β isoforms and their receptors in acute and chronic experimental diabetes in rats. Endocrinology. 2000. 141:1196–1208.
Article22. Border WA, Nobel NA. Transforming growth factor β in tissue in tissue fibrosis. N Engl J Med. 1994. 331:1286–1292.23. Branton MH, Kopp JB. TGF-β and fibrosis. Microbes Infect. 1999. 15:1349–1365.
Article24. Border WA, Noble NA. TGF-β in kidney fibrosis: a target for gene therapy. Kidney Int. 1997. 51:1388–1396.
Article25. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998. 391:806–811.
Article26. Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrsl JH, Fauci AS. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986. 83:4167–4171.27. Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 2005. 67:1762–1771.
Article28. Baricos WH, Reed JC, Cortez SL. Extracellular matrix degradation by cultured mesangial cells: Mediators and modulators. Exp Biol Med. 2003. 228:1018–1022.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Attenuation of the Expression of Fibrogenic Molecules by Transforming Growth Factor-beta1 Antisense in Cultured Rat Mesangial Cells
- Aldosterone-induced TGF-beta1 Expression is Regulated by Mitogen-Activated Protein Kinases and Activator Protein-1 in Mesangial Cells
- Effect of Antisense TGF-beta1 Oligodeoxynucleotides in Streptozotocin-Induced Diabetic Rat Kidney
- Monocyte Chemoattractant Protein-1 Upregulates Fibronectin Secretion by Human Peritoneal Fibroblasts
- Antifibrotic Effect of BMP-7 in the Peritoneum and the Mechanism