Transplantation of Neural Differentiated Human Mesenchymal Stem Cells into the Cochlea of an Auditory-neuropathy Guinea Pig Model
- Affiliations
-
- 1Department of Otolaryngology, Chonnam National University Medical School, Gwangju, Korea. victocho@hanmail.net
- 2Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- 3Brain Korea 21 Project, Center for Biomedical Human Resources at Chonnam National University, Gwangju, Korea.
- 4Research Institute of Medical Sciences, Chonnam National University, Gwangju, Korea.
- KMID: 1777878
- DOI: http://doi.org/10.3346/jkms.2011.26.4.492
Abstract
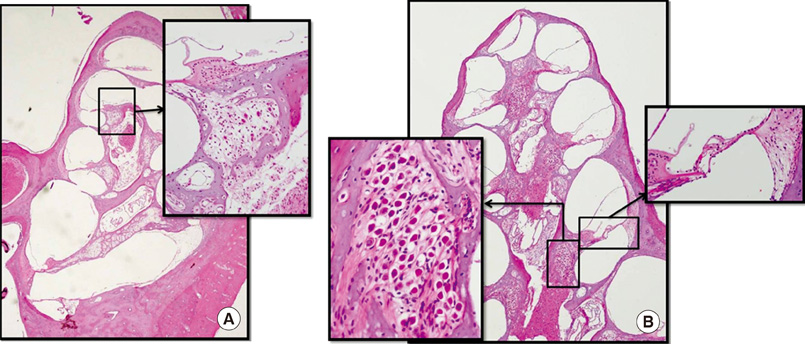
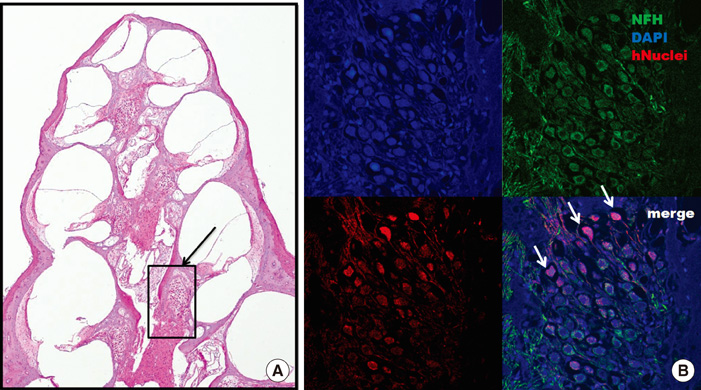
- The aim of this study was to determine the effects of transplanted neural differentiated human mesenchymal stem cells (hMSCs) in a guinea pig model of auditory neuropathy. In this study, hMSCs were pretreated with a neural-induction protocol and transplanted into the scala tympani of the guinea pig cochlea 7 days after ouabain injury. A control model was made by injection of Hanks balanced salt solution alone into the scala tympani of the guinea pig cochlea 7 days after ouabain injury. We established the auditory neuropathy guinea pig model using 1 mM ouabain application to the round window niche. After application of ouabain to the round window niche, degeneration of most spiral ganglion neurons (SGNs) without the loss of hair cells within the organ of Corti and increasing the auditory brain responses (ABR) threshold were found. After transplantation of neural differentiated hMSCs, the number of SGNs was increased, and some of the SGNs expressed immunoreactivity with human nuclear antibody under confocal laser scanning microscopy. ABR results showed mild hearing recovery after transplantation. Based on an auditory neuropathy animal model, these findings suggest that it may be possible to replace degenerated SGNs by grafting stem cells into the scala tympani.
MeSH Terms
-
Animals
Cardiotonic Agents/toxicity
Cochlea/drug effects/pathology
Disease Models, Animal
Female
Guinea Pigs
Hearing Loss, Central/chemically induced/pathology/*therapy
Humans
*Mesenchymal Stem Cell Transplantation
Mesenchymal Stem Cells/*cytology
Neurogenesis
Ouabain/toxicity
Spiral Ganglion/pathology
Transplantation, Heterologous
Figure
Cited by 3 articles
-
Regenerative Cell Therapy for the Sensorineural Hearing Loss
Kyoung Ho Park
Hanyang Med Rev. 2015;35(2):113-120. doi: 10.7599/hmr.2015.35.2.113.Therapeutic Efficacy of Bone Marrow Derived Mesenchymal Stem Cells in Ototoxic Sensorineural Hearing Loss
Subin Kim, Yoon Hee Kwon, In Beom Kim, Young Jun Seo, Jae Sang Han, Jae-Hyun Seo, Hyun Jin Lee, Kyoung Ho Park
Korean J Otorhinolaryngol-Head Neck Surg. 2020;63(12):564-569. doi: 10.3342/kjorl-hns.2020.00759.Neural-Induced Human Mesenchymal Stem Cells Promote Cochlear Cell Regeneration in Deaf Guinea Pigs
Sujeong Jang, Hyong-Ho Cho, Song-Hee Kim, Kyung-Hwa Lee, Jae Yeoul Jun, Jong-Seong Park, Han-Seong Jeong, Yong-Beom Cho
Clin Exp Otorhinolaryngol. 2015;8(2):83-91. doi: 10.3342/ceo.2015.8.2.83.
Reference
-
1. Suzuka Y, Schuknecht HF. Retrograde cochlear neuronal degeneration in human subjects. Acta Otolaryngol Suppl. 1988. 450:1–20.2. Keithley EM, Croskrey KL. Spiral ganglion cell endings in the cochlear nucleus of young and old rats. Hear Res. 1990. 49:169–177.3. Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol. 2000. 11:215–230.4. Lang H, Schulte BA, Goddard JC, Hedrick M, Schulte JB, Wei L, Schmiedt RA. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008. 9:225–240.5. Schmiedt RA, Okamura HO, Lang H, Schulte BA. Ouabain application to the round window of the gerbil cochlea: a model of auditory neuropathy and apoptosis. J Assoc Res Otolaryngol. 2002. 3:223–233.6. Iguchi F, Nakagawa T, Tateya I, Kim TS, Endo T, Taniguchi Z, Naito Y, Ito J. Trophic support of mouse inner ear by neural stem cell transplantation. Neuroreport. 2003. 14:77–80.7. Shinohara T, Bredberg G, Ulfendahl M, Pyykkö I, Olivius NP, Kaksonen R, Lindström B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002. 99:1657–1660.8. Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000. 1:315–325.9. Li H, Corrales CE, Edge A, Heller S. Stem cells as therapy for hearing loss. Trends Mol Med. 2004. 10:309–315.10. Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci U S A. 2003. 100:13495–13500.11. Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Géléoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007. 8:18–31.12. Parker MA, Cotanche DA. The potential use of stem cells for cochlear repair. Audiol Neurootol. 2004. 9:72–80.13. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001. 282:148–152.14. Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004. 113:1701–1710.15. Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, Kusano R, Nakagawa S, Satoh H, Fujii M, Matsunaga T. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007. 171:214–226.16. Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007. 117:1629–1635.17. Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H. Neural induction with neurogenin 1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008. 26:2217–2228.18. Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, Park IH, Kim SU. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007. 2:e1272.19. Cho HH, Jeong HS, Jang SJ, Park JS, Cho HC, Jang CH, Cho YB. Neural differentiation of mesenchymal stem cells from bone marrow of human mastoid process. Korean J Otorhinolaryngol-Head Neck Surg. 2008. 51:422–428.20. Cho HH, Jang S, Lee SC, Jeong HS, Park JS, Han JY, Lee KH, Cho YB. Effect of neural-induced mesenchymal stem cells and platelet-rich plasma on facial nerve regeneration in an acute nerve injury model. Laryngoscope. 2010. 120:907–913.21. Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010. 120:29–40.22. Nakayama D, Matsuyama T, Ishibashi-Ueda H, Nakagomi T, Kasahara Y, Hirose H, Kikuchi-Taura A, Stern DM, Mori H, Taguchi A. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur J Neurosci. 2010. 31:90–98.23. Tamura T, Nakagawa T, Iguchi F, Tateya I, Endo T, Kim TS, Dong Y, Kita T, Kojima K, Naito Y, Omori K, Ito J. Transplantation of neural stem cells into the modiolus of mouse cochleae injured by cisplatin. Acta Otolaryngol Suppl. 2004. 551:65–68.24. Lee JE, Nakagawa T, Kim TS, Iguchi F, Endo T, Dong Y, Yuki K, Naito Y, Lee SH, Ito J. A novel model for rapid induction of apoptosis in spiral ganglions of mice. Laryngoscope. 2003. 113:994–999.25. Shiga A, Nakagawa T, Nakayama M, Endo T, Iguchi F, Kim TS, Naito Y, Ito J. Aging effects on vestibulo-ocular responses in C57BL/6 mice: comparison with alteration in auditory function. Audiol Neurootol. 2005. 10:97–104.26. Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002. 22:275–279.27. Coleman B, de Silva MG, Shepherd RK. Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells. 2007. 25:2685–2694.28. Nadol JB Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT Jr, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001. 110:883–891.29. Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003. 101:3722–3729.30. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003. 75:389–397.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neural-Induced Human Mesenchymal Stem Cells Promote Cochlear Cell Regeneration in Deaf Guinea Pigs
- Isolation, Characterization and Growth Kinetic Comparison of Bone Marrow and Adipose Tissue Mesenchymal Stem Cells of Guinea Pig
- Isolation and Culture of Adult Neural Stem Cells from Guinea Pig Tympanic Membrane
- Auditory Effects of Microperfused Lidocaine on Guinea Pig Cochlea
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy