J Breast Cancer.
2007 Jun;10(2):127-133. 10.4048/jbc.2007.10.2.127.
The Effects of Genistein to Expression of Fatty Acid Synthase in Breast Cancer Cells
- Affiliations
-
- 1Department of Surgery, Koyang University Hospital, Daejeon, Korea. dsyoonmd@kyuh.co.kr
- 2Department of Urology, Koyang University Hospital, Daejeon, Korea.
- 3Myung-gok Research Institute for Medical Science, Koyang University Hospital, Daejeon, Korea.
- KMID: 1745053
- DOI: http://doi.org/10.4048/jbc.2007.10.2.127
Abstract
-
PURPOSE: The relatively low incidence of breast cancer in Asian countries with cultures which traditionally eat a large amount of soy is worth noticing in research fields. Genistein is a isoflavone phytoestrogen found in soy and its consumption may have a role in cancer etiology. We have established a hypothesis that a diet high in soy consumption is related to a low incidence of breast cancer. Fatty acid synthase (FAS) is a multi-protein enzyme responsible for de novo biosynthesis of fatty acids. Recent studies have demonstrated that high levels of FAS occurs in a subset of human cancers, such as breast cancer, ovarian cancer, and prostate cancer. High level of FAS are associated with a poor prognosis. Sterol regulatory element binding proteins (SREBPs) are a family of transcription factors that regulate genes involved in lipid metabolism, including FAS. Recent studies show that expression of SREBP1c is correlates with FAS expression. The aim of this study is to investigate the effect of genistein on the expression of FAS in breast cancer cells.
METHODS
We performed immunofluorescent staining to examine the expression of FAS under different concentration of genistein. RT-PCR was also performed to investigate the mRNA expression of FAS and SREBP1c in different conditioned breast cancer cells treated with different concentration of FAS inhibitor and genistein.
RESULTS
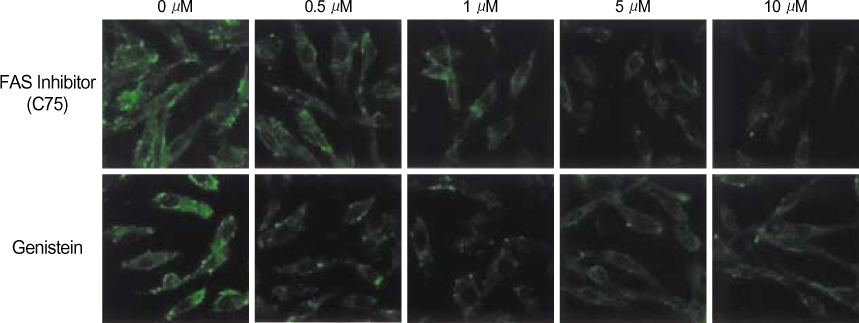
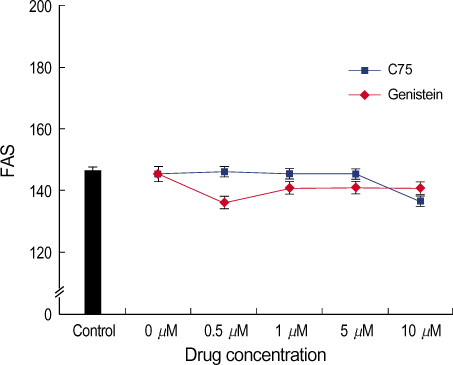
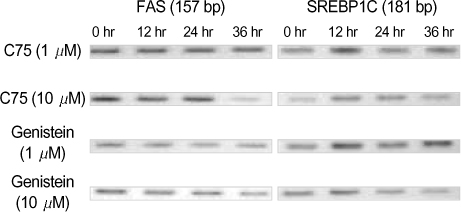
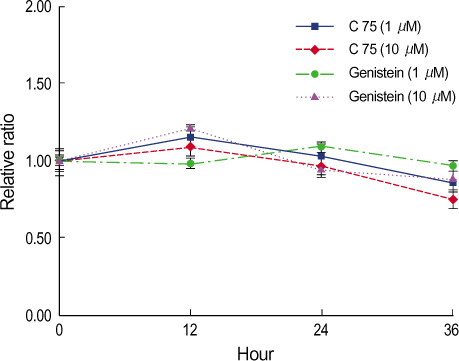
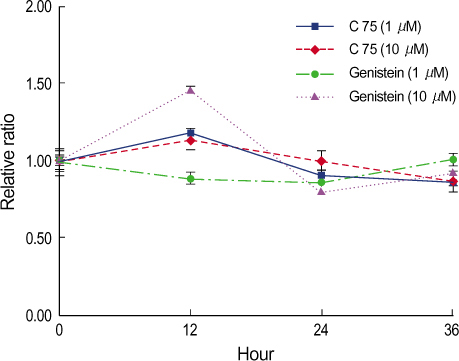
By immunofluorescent staining, the FAS expression after treatment with the FAS inhibitor, C75, decreased at a micron10 M concentration. However the expression of FAS decreased at all concentrations of genistein (0.5, 1, 5, 10 micronM). The mRNA levels of FAS and SREBP1c after treatment with C75 decreased constantly according to time and concentration. However the effect was noted only after 12 hr. The mRNA level of FAS and SREBP1c following treatment with genistein decreased at only a 10 micronM concentration (p<0.005).
CONCLUSION
Genistein may down regulate FAS expression in breast cancer cells through modulation of SREBP-1c. This finding may account for the relatively low incidence of breast cancer in Asians who consume a large amount of soy in their diet.
Keyword
MeSH Terms
-
Asian Continental Ancestry Group
Breast Neoplasms*
Breast*
Diet
Fatty Acids
Genistein*
Humans
Incidence
Lipid Metabolism
Ovarian Neoplasms
Phytoestrogens
Prognosis
Prostatic Neoplasms
RNA, Messenger
Sterol Regulatory Element Binding Protein 1
Sterol Regulatory Element Binding Proteins
Transcription Factors
Fatty Acids
Genistein
Phytoestrogens
RNA, Messenger
Sterol Regulatory Element Binding Protein 1
Sterol Regulatory Element Binding Proteins
Transcription Factors
Figure
Reference
-
1. Ministry of Health and Welfare. Annual Report of the Korea Central Cancer Registry 2003.2. Menendez JA, Decker JP, Lupu R. In support Fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005. 94:1–4.
Article3. Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA. 1994. 91:6379–6383.
Article4. Yang Y, Morin PJ, Han WF, Chen T, Bornman DM, Gabrielson EW, et al. Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element biding protein-1c. Exp Cell Res. 2003. 282:132–137.
Article5. Menendez JA, Lupu R, Colomer R. Targeting fatty acid synthase: potential for therapeutic intervention in Her-2/neu-overexpressing breast cancer. Drug News Perspect. 2005. 18:375–385.
Article6. Koo TY, Go DK, Choi IS, Choi WJ, Yoon DS, Sul HJ, et al. FAS expression in breast cancer. J Breast Cancer. 2005. 8:40–44.7. Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci USA. 2004. 101:10715–10720.
Article8. Sonoda T, Nagata Y, Mori M, Miyanaga N, Takashima N, Okumura K, et al. A case-control study of diet and prostate cancer in Japan: potential protective effect of traditional Japanese diet. Cancer Sci. 2004. 95:238–242.
Article9. Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci USA. 2000. 97:3450–3454.
Article10. Menendez JA, Vellon L, Colomer R, Lupu R. Pharmacological & small interference RNA-mediated inhibition of breast cancer associated fattyacid synthase (oncogenic antigen-519) synergistically enhances Taxol (packitaxel)-induced cytotoxicity. Int J Cancer. 2005. 115:19–35.
Article11. Martel PM, Bingham CM, McGraw CJ, Baker CL, Morganelli PM, Meng ML, et al. S14 protein in breast cancer cells: superinduction with progestin, and effects on cell growth. Exp Cell Res. 2006. 312:278–288.12. Sebastiani V, Visca P, Botti C, Santeusanio G, Galati GM, Piccini V, et al. Fatty acid synthase is a marker od increased risk of recurrence in endometrial carcinoma. Gynecol Oncol. 2004. 92:101–105.
Article13. Shon YH, Park SD, Nam KS. Effective chemopreventive activity of genistein against human breast cancer cells. J Biochem Mol Biol. 2006. 39:448–451.
Article14. Visca P, Alo PL, Nonno FD, Botti C, Trombetta G, Marandino F, et al. Immunohistochemical expression of fatty acid synthase, apoptotic regulating genes, proliferating factors, and ras protein product in colorectal adenomas, carcinomas and adjacent nonneoplastic mucosa. Clin Cancer Res. 1999. 5:4111–4118.15. Liu B, Wang Y, Fillgrove KL, Anderson VE. Triclosan inhibits enoyl-reductase of type I fatty acid synthase in vitro and is cytotoxic to MCF-7 and SKBr-3 breast cancer cells. Cancer Chemother Pharmacol. 2002. 49:187–193.
Article16. Boizard M, Le Liepvre X, Lemarchand P, Foufelle F, Ferre P, Dugail I. Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J Biol Chem. 1998. 273:29164–29171.
Article17. Liu B, Edgerton S, Yang X, Kim A, Ordonez-Ercan D, Mason T, et al. Low dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res. 2005. 65:879–886.
Article18. Chen WF, Wong MS. Genistein enhances insulin-like growth factor signaling pahway in human breast cancer (MCF-7) cells. J Clin Endocrinol Metab. 2004. 89:2351–2359.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- FAS (Fatty acid synthase) expression in breast cancer
- Different Expression of p27(kip1) and p57(kip2) by Genistein Treatment in MDA-MB-231 Human Breast Cancer Cells
- Inhibition of Cyclooxygenase-2 (Cox-2) Expression by Genistein in Breast Cancer Cell-line
- Effects of Genistein Supplementation on Fatty Liver and Lipid Metabolism in Rats Fed High Fat Diet
- Molecular Mechanism of the G2/M Arrest in Breast Cancer Cell Lines (T47D and MDA-MB231) Induced by Genistein