J Vet Sci.
2013 Sep;14(3):345-353. 10.4142/jvs.2013.14.3.345.
Black rice anthocyanidins prevent retinal photochemical damage via involvement of the AP-1/NF-kappaB/Caspase-1 pathway in Sprague-Dawley Rats
- Affiliations
-
- 1Department of Public Health, Chengdu Medical College, Chengdu City, 610050, China. cyggwsyxp@sina.com
- 2Department of Nutrition and Food Hygiene, School of Preventive Medicine, The Third Military Medical University, Chongqing City, 400038, China.
- 3Department of Epidemiology and Bio-statistics, School of Public Health, Drexel University, Philadelphia, PA 19102, USA.
- KMID: 1705566
- DOI: http://doi.org/10.4142/jvs.2013.14.3.345
Abstract
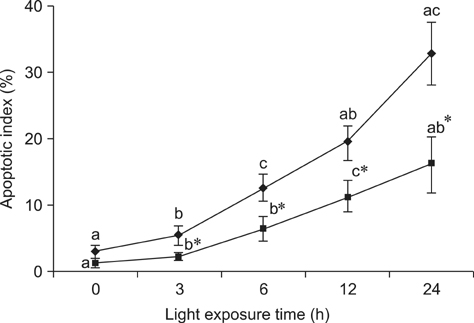
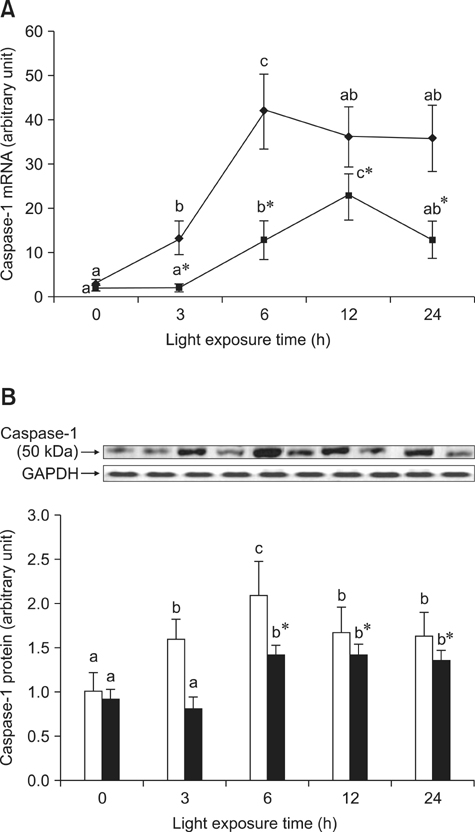
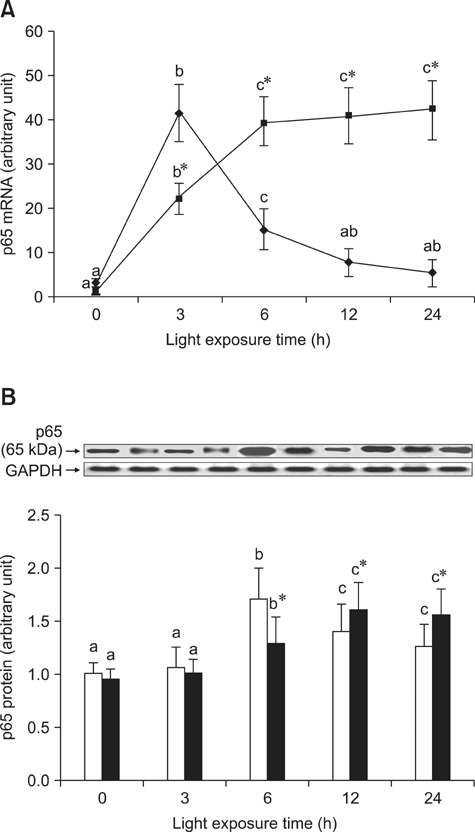
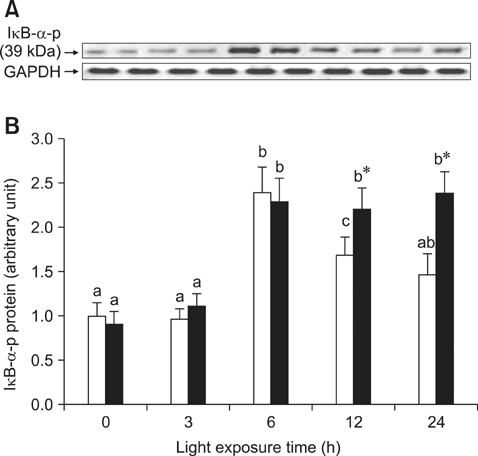
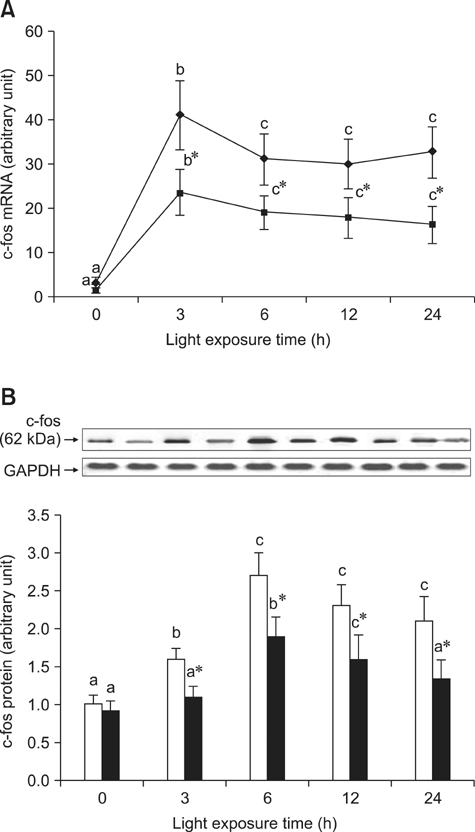
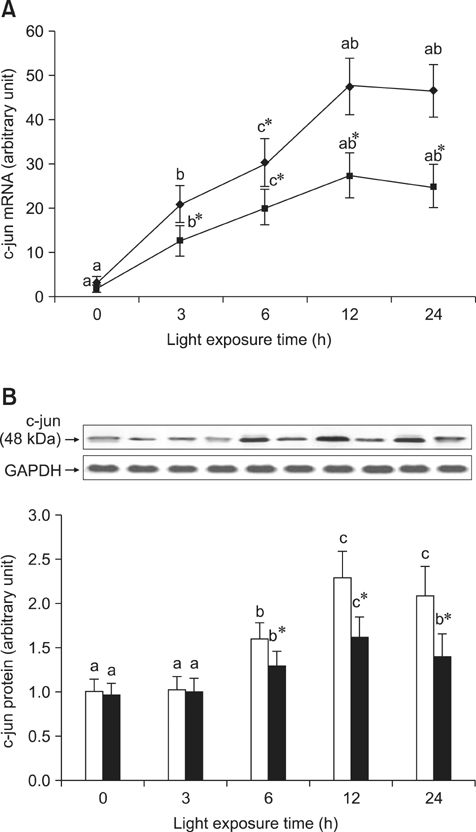
- The effects of black rice anthocyanidins (BRACs) on retinal damage induced by photochemical stress are not well known. In the present study, Sprague-Dawley rats were fed AIN-93M for 1 week, after which 80 rats were randomly divided into two groups and treated with (n = 40) or without BRACs (n = 40) for 15 days, respectively. After treatment, both groups were exposed to fluorescent light (3,000 +/- 200 lux; 25degrees C), and the protective effect of dietary BRACs were evaluated afterwards. Our results showed that dietary BRACs effectively prevented retinal photochemical damage and inhibited the retinal cells apoptosis induced by fluorescent light (p < 0.05). Moreover, dietary BRACs inhibited expression of AP-1 (c-fos/c-jun subunits), up-regulated NF-kappaB (p65) expression and phosphorylation of IkappaB-alpha, and decreased Caspase-1 expression (p < 0.05). These results suggest that BRACs improve retinal damage produced by photochemical stress in rats via AP-1/NF-kappaB/Caspase-1 apoptotic mechanisms.
MeSH Terms
-
Animal Feed/analysis
Animals
Anthocyanins/administration & dosage/*pharmacology
Antioxidants/administration & dosage/*physiology
Blotting, Western
Caspase 1/*genetics/metabolism
Diet
Dietary Supplements/analysis
I-kappa B Proteins/genetics/metabolism
NF-kappa B/*genetics/metabolism
Neoplasm Proteins/genetics/metabolism
Nucleocytoplasmic Transport Proteins/genetics/metabolism
Oryza sativa/chemistry
Proto-Oncogene Proteins c-fos/genetics/metabolism
Proto-Oncogene Proteins c-jun/genetics/metabolism
Rats
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
Retinal Diseases/etiology/*prevention & control
Signal Transduction/*drug effects/radiation effects
Transcription Factor AP-1/*genetics/metabolism
Anthocyanins
Antioxidants
Caspase 1
I-kappa B Proteins
NF-kappa B
Neoplasm Proteins
Nucleocytoplasmic Transport Proteins
Proto-Oncogene Proteins c-fos
Proto-Oncogene Proteins c-jun
Transcription Factor AP-1
Figure
Reference
-
1. Aonuma H, Yamazaki R, Watanabe I. Retinal cell death by light damage. Jpn J Ophthalmol. 1999; 43:171–179.
Article2. Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999; 18:6910–6924.
Article3. Büchi ER, Szczesny PJ. Necrosis and apoptosis in neuroretina and pigment epithelium after diffuse photodynamic action in rats: a light and electron microscopic study. Jpn J Ophthalmol. 1996; 40:1–11.4. Chahory S, Padron L, Courtois Y, Torriglia A. The LEI/L-DNase II pathway is activated in light-induced retinal degeneration in rats. Neurosci Lett. 2004; 367:205–209.
Article5. Chandra A, Rana J, Li Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. J Agric Food Chem. 2001; 49:3515–3521.
Article6. Cichocki M, Paluszczak J, Szaefer H, Piechowiak A, Rimando AM, Baer-Dubowska W. Pterostilbene is equally potent as resveratrol in inhibiting 12-O-tetradecanoylphorbol-13-acetate activated NFκB, AP-1, COX-2, and iNOS in mouse epidermis. Mol Nutr Food Res. 2008; 52:S62–S70.7. Crawford MJ, Krishnamoorthy RR, Rudick VL, Collier RJ, Kapin M, Aggarwal BB, Al-Ubaidi MR, Agarwal N. Bcl-2 overexpression protects photooxidative stress-induced apoptosis of photoreceptor cells via NF-κB preservation. Biochem Biophys Res Commun. 2001; 281:1304–1312.
Article8. Donovan M, Carmody RJ, Cotter TG. Light-induced photoreceptor apoptosis in vivo requires neuronal nitric-oxide synthase and guanylate cyclase activity and is caspase-3-independent. J Biol Chem. 2001; 276:23000–23008.
Article9. Geller AM, Sutton LD, Marshall RS, Hunter DL, Madden V, Peiffer RL. Repeated spike exposure to the insecticide chlorpyrifos interferes with the recovery of visual sensitivity in rats. Doc Ophthalmol. 2005; 110:79–90.
Article10. Grimm C, Wenzel A, Hafezi F, Remé CE. Gene expression in the mouse retina: the effect of damaging light. Mol Vis. 2000; 6:252–260.11. Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Remé CE. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat Genet. 2000; 25:63–66.
Article12. Hafezi F, Grimm C, Wenzel A, Abegg M, Yaniv M, Remé CE. Retinal photoreceptors are apoptosis-competent in the absence of JunD/AP-1. Cell Death Differ. 1999; 6:934–936.13. Hafezi F, Steinbach JP, Marti A, Munz K, Wang ZQ, Wagner EF, Aguzzi A, Remé CE. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med. 1997; 3:346–349.
Article14. Jang YP, Zhou J, Nakanishi K, Sparrow JR. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem Photobiol. 2005; 81:529–536.
Article15. Kalt W, Hanneken A, Milbury P, Tremblay F. Recent research on polyphenolics in vision and eye health. J Agric Food Chem. 2010; 58:4001–4007.
Article16. Kaltschmidt B, Uherek M, Wellmann H, Volk B, Kaltschmidt C. Inhibition of NF-κB potentiates amyloid β-mediated neuronal apoptosis. Proc Natl Acad Sci U S A. 1999; 96:9409–9414.
Article17. Kowalczyk E, Krzesiński P, Kura M, Szmigiel B, Błaszczyk J. Anthocyanins in medicine. Pol J Pharmacol. 2003; 55:699–702.18. Krishnamoorthy RR, Crawford MJ, Chaturvedi MM, Jain SK, Aggarwal BB, Al-Ubaidi MR, Agarwal N. Photo-oxidative stress down-modulates the activity of nuclear factor-κB via involvement of caspase-1, leading to apoptosis of photoreceptor cells. J Biol Chem. 1999; 274:3734–3743.
Article19. Li F, Cao W, Anderson RE. Alleviation of constant-light-induced photoreceptor degeneration by adaptation of adult albino rat to bright cyclic light. Invest Ophthalmol Vis Sci. 2003; 44:4968–4975.
Article20. Liu Y, Song X, Han Y, Zhou F, Zhang D, Ji B, Hu J, Lv Y, Cai S, Wei Y, Gao F, Jia X. Identification of anthocyanin components of wild Chinese blueberries and amelioration of light-induced retinal damage in pigmented rabbit using whole berries. J Agric Food Chem. 2011; 59:356–363.
Article21. Matsumoto H, Nakamura Y, Iida H, Ito K, Ohguro H. Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp Eye Res. 2006; 83:348–356.
Article22. Matsunaga N, Imai S, Inokuchi Y, Shimazawa M, Yokota S, Hara H. Bilberry and its main constituents have neuroprotective effects against retinal neuronal damage in vitro and in vivo. Mol Nutr Food Res. 2009; 53:869–877.
Article23. Mattson MP, Camandola S. NF-κB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001; 107:247–254.
Article24. McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res. 2007; 51:702–713.
Article25. Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966; 5:450–473.26. Organisciak DT, Bicknell IR, Darrow RM. The effects of L-and D-ascorbic acid administration on retinal tissue levels and light damage in rats. Curr Eye Res. 1992; 11:231–241.
Article27. Ranchon I, Gorrand JM, Cluzel J, Droy-Lefaix MT, Doly M. Functional protection of photoreceptors from light-induced damage by dimethylthiourea and Ginkgo biloba extract. Invest Ophthalmol Vis Sci. 1999; 40:1191–1199.28. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.
Article29. Stoyanovsky DA, Goldman R, Darrow RM, Organisciak DT, Kagan VE. Endogenous ascorbate regenerates vitamin E in the retina directly and in combination with exogenous dihydrolipoic acid. Curr Eye Res. 1995; 14:181–189.
Article30. Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000; 69:217–245.
Article31. Tanaka J, Nakanishi T, Ogawa K, Tsuruma K, Shimazawa M, Shimoda H, Hara H. Purple rice extract and anthocyanidins of the constituents protect against light-induced retinal damage in vitro and in vivo. J Agric Food Chem. 2011; 59:528–536.
Article32. Torres J, Enríquez-de-Salamanca A, Fernández I, Rodríguez-Ares MT, Quadrado MJ, Murta J, del Castillo JMB, Stern ME, Calonge M. Activation of MAPK signaling pathway and NF-κB activation in pterygium and ipsilateral pterygium-free conjunctival specimens. Invest Ophthalmol Vis Sci. 2011; 52:5842–5852.
Article33. Wenzel A, Grimm C, Marti A, Kueng-Hitz N, Hafezi F, Niemeyer G, Remé CE. c-fos Controls the "private pathway" of light-induced apoptosis of retinal photoreceptors. J Neurosci. 2000; 20:81–88.
Article34. Wenzel A, Grimm C, Samardzija M, Remé CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005; 24:275–306.
Article35. Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006; 51:461–481.
Article36. Wu T, Chen Y, Chiang SKS, Tso MOM. NF-κB activation in light-induced retinal degeneration in a mouse model. Invest Ophthalmol Vis Sci. 2002; 43:2834–2840.37. Wu T, Chiang SKS, Chau FY, Tso MOM. Light-induced photoreceptor degeneration may involve the NFκB/caspase-1 pathway in vivo. Brain Res. 2003; 967:19–26.
Article38. Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, Li Y, Chi D, Yu X, Zhao T, Han P, Xia X, Ling W. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor γ-liver X receptor α-ABCA1 pathway. J Biol Chem. 2005; 280:36792–36801.
Article39. Xia M, Ling WH, Ma J, Kitts DD, Zawistowski J. Supplementation of diets with the black rice pigment fraction attenuates atherosclerotic plaque formation in apolipoprotein e deficient mice. J Nutr. 2003; 133:744–751.
Article40. Yu X, Chen K, Wei N, Zhang Q, Liu J, Mi M. Dietary taurine reduces retinal damage produced by photochemical stress via antioxidant and anti-apoptotic mechanisms in Sprague-Dawley rats. Br J Nutr. 2007; 98:711–719.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NF-kappa B activation following optic nerve transection
- PDTC Inhibits TNF-alpha-Induced Apoptosis in MC3T3E1 Cells
- The Impact of Kinds of Dietary Grain and Dietary Lipid Level on the Glucose Metabolism and Antithrombogenic Capacity of Full Grown Obesity Induced Rats
- Alteration of PKC, PKC theta, NF-kappa B and AP-1 in Ischemic-reperfused Soleus Muscle of Rat with Ischemic Preconditioning
- NF-kappaB Binding Activity and Cyclooxygenase-2 Expression in Persistent betaCCI(4)-Treated Rat Liver Injury