J Korean Soc Endocrinol.
2006 Apr;21(2):106-115. 10.3803/jkes.2006.21.2.106.
Transcriptional Regulation of the Estrogen Receptor alpha Gene by Testosterone in Cultures of Primary Rat Sertoli Cells
- Affiliations
-
- 1Department of Urology, College of Medicine, Konkuk University, Korea.
- 2Daejeon Health Sciences College, Korea.
- 3Department of Biochemistry, College of Medicine, Chungnam National University, Korea.
- 4Cancer Research Institute, Chungnam National University, Korea.
- KMID: 1511949
- DOI: http://doi.org/10.3803/jkes.2006.21.2.106
Abstract
-
BACKGROUND: We wanted to identify the presence of the estrogen receptor (ER) alpha in Sertoli cells and gain insight on the regulation of the ER alpha gene expression by testosterone in Sertoli cells. The transcriptional regulation of the ER alpha gene was investigated in primary Sertoli cell cultures by in situ hybridization and reverse transcription-polymerase chain reaction (RT-PCR).
METHODS
Primary Sertoli cell culture was performed. The expression levels of ER alpha and ER beta mRNA in Sertoli cells were detected by Northern blot, RT-PCR, immunocytochemistry and in situ hybridization.
RESULTS
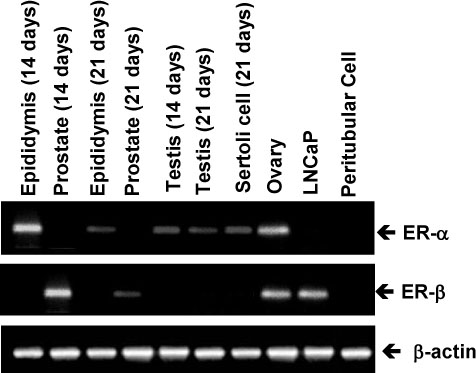
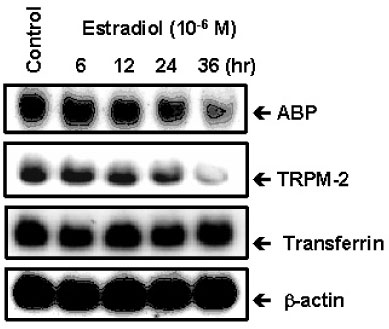
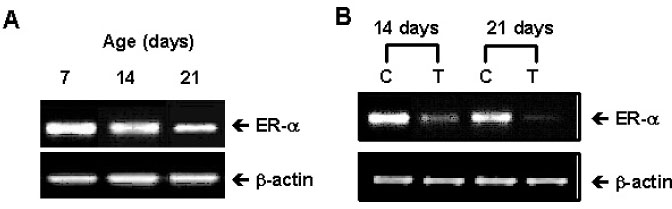
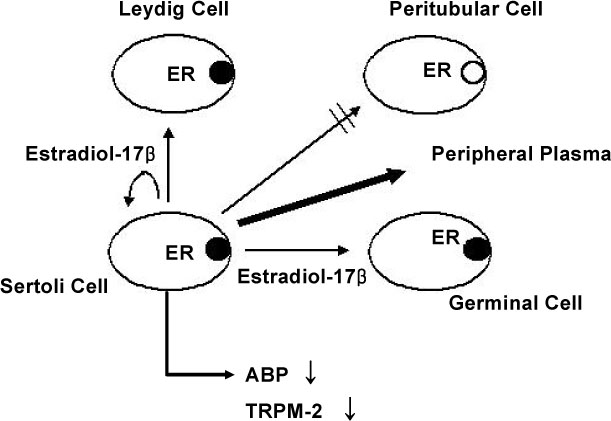
The ovary, testis and epididymis showed a moderate to high expression of ER alpha while the prostate, ovary and LNCap cells showed the ER beta expression. ER alpha mRNA and protein were detected in the germ cells and Sertoli cells by in situ hybridization and immunocytochemistry. The level of ER alpha mRNA was gradually decreased in a time-dependent manner after testosterone treatment, and the changes of ER alpha mRNA were dependent on the concentration of testosterone. Androgen binding protein and testosterone-repressive prostate message-2 (TRPM-2) mRNA were reduced at 24 hour by estradiol, while the transferrin mRNA was not affected. ER alpha mRNA was strongly detectable in the testes of 7 days-old-rats, but it was gradually decreased from 14 to 21 days of age. The primary Sertoli cells also showed the same pattern. The ER alpha gene expression was also regulated by testosterone in the Sertoli cells prepared from the 14- and 21-day old rats.
CONCLUSIONS
These results suggest that ER alpha is transcriptionally regulated by testosterone and it may play some role in the Sertoli cells.
MeSH Terms
-
Androgen-Binding Protein
Animals
Blotting, Northern
Cell Culture Techniques
Epididymis
Estradiol
Estrogen Receptor alpha*
Estrogens*
Female
Gene Expression
Germ Cells
Immunohistochemistry
In Situ Hybridization
Male
Ovary
Prostate
Rats*
RNA, Messenger
Sertoli Cells*
Testis
Testosterone*
Transferrin
Androgen-Binding Protein
Estradiol
Estrogen Receptor alpha
Estrogens
RNA, Messenger
Testosterone
Transferrin
Figure
Reference
-
1. Means AR, Dedman JR, Tash JS, Tindall DJ, van Sickle M, Welsh MJ. Regulation of the testis Sertoli cell by follicle stimulating hormone. Ann Rev Physiol. 1980. 42:59–70.2. Skinner MK, Schitz SM, Anthony CT. Regulation of Sertoli cell differentiated function: Testicular transferrin and androgen-binding protein expression. Endocrinology. 1989. 124:3015–3024.3. Skinner MK, Griswold MD. Sertoli cells synthesize and secrete a ceruloplasmin-like protein. Biol Reprod. 1983. 28:1225–1229.4. Joseph DR, Hall SH, French FS. Identification of complementary DNA clones that encode rat androgen binding protein. J Androl. 1985. 6:392–395.5. Lim K, Yoon SJ, Lee MS, Byun SH, Kweon GR, Kwak ST, Hwang B. Glucocorticoid regulation of androgen binding protein expression in primary Sertoli cell cultures from rats. Biochem Biophys Res Commun. 1996. 17:490–494.6. Hall SH, Joseph DR, French FS, Conti M. Follicle-stimulating hormone induces transient expression of the protooncogene c-fos in primary Sertoli cell cultures. Mol Endocrinol. 1988. 2:55–61.7. Lim K, Yoo JH, Kim KY, Kweon GR, Kwak ST, Hwang BD. Testosterone regulation of protooncogene c-myc expression in primary Sertoli cell cultures from prepubertal rats. J Androl. 1994. 15:543–550.8. Lim K, Hwang BD. Follicle-stimulating hormone transiently induces expression of protooncogene c-myc in primary Sertoli cell cultures of early pubertal and prepubertal rat. Mol Cell Endocrinol. 1995. 111:51–56.9. Lim K, Lee JI, Kwak ST, Lee MS, Hwang BD. Testosterone downregulates expression of the β-nerve growth factor receptor gene in primary Sertoli cell cultures. Kor J Biochem. 1994. 26:91–98.10. Morris PL, Vale WW, Cappel S, Bardin CW. Inhibin production by primary Sertoli cell enriched cultures: Regulation by follicle-stimulating hormone, androgens and epidermal growth factor. Endocrinology. 1988. 122:717–725.11. Smith EP, Dickson BA, Chernausek SD. Insulin-like growth factor binding protein-3 secretion from cultured rat Sertoli cells: dual regulation by follicle stimulating hormone and insulin-like growth factor-I. Endocrinology. 1990. 127:2744–2751.12. Skinner MK, Takacs K, Coffey RJ. Transforming growth factor-alpha gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Endocrinology. 1989. 124:845–854.13. Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994. 266:1524–1527.14. Baird DT, Galbraith A, Fraser IS, Newsam JE. The concentration of oestrone and oestradiol 17-β in the spermatic venous blood in man. J Endocrinol. 1973. 57:285–288.15. Dorrington JH, Khan SA. Russell LD, Griswold MD, editors. Steroid production, metabolism and release by Sertoli cells. The Sertoli cell. 1993. Clearwater: Cache River Press;538–549.16. Dorrington JH, Roller NF, Fritz IB. Effects of follicle stimulating hormone on cultures of Sertoli cell preparations. Mol Cell Endocrinol. 1975. 3:57–70.17. Huggenvik J, Idzerda RL, Haywood L, Lee DC, McKnight GS, Griswold MD. Transferrin messenger ribonucleic acid: molecular cloning and hormonal regulation in rat Sertoli cells. Endocrinology. 1987. 120:332–340.19. Pena SD. A new technique for the visualization of the cytoskeleton in cultured fibroblasts with Coomassie blue R250. Cell Biol Int Rep. 1980. 4:149–153.20. Virca GD, Northemann W, Shiels BR, Widera G, Broome S. Simplified Northern blot hybridization using 5% sodium dodecyl sulfate. Biotechniques. 1990. 8:370–371.21. Collard MW, Griswold MD. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987. 26:3297–3303.22. Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983. 132:6–13.23. Grandien K, Backdahl M, Ljunggren O, Gustafsson JA, Berkenstam A. Estrogen target tissue determines alternative promoter utilization of human estrogen receptor gene in osteoblasts and tumor cell lines. Endocrinology. 1995. 136:2223–2229.24. Van Etten RA, Jackson P, Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989. 58:669–678.25. Heiles HB, Genersch E, Kessler C, Neumann R, Eggers HJ. In situ hybridization with digoxigenin-labeled DNA of human papillomaviruses (HPV 16/18) in HeLa and SiHa cells. Biotechniques. 1988. 6:978–981.26. Verhoeven G, Cailleau J. Prolonged exposure to androgens suppresses follicle-stimulating hormone-induced aromatase activity in rat Sertoli cell cultures. Mol Cell Endocrinol. 1988. 57:51–60.27. Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997. 138:863–870.28. Albrecht ED, Billiar RB, Aberdeen GW, Babischkin JS, Pepe GJ. Expression of estrogen receptors alpha and beta in the fetal baboon testis and epididymis. Biol Reprod. 2004. 70:1106–1113.29. Koike S, Sakai M, Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic Acids Res. 1987. 15:2499–2513.30. O'donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocrinol Rev. 2001. 22:289–318.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of growth factor receptor HER2 on the estrogen receptor transcriptional activities and its implications
- Effects of estrogen receptor and estrogen on the chromatin structure in estrogen receptor stable transfectants
- Regulation of Estrogen Receptor mRNA in Rat Anterior Pituitary Gland
- Bone Morphogenetic Protein-2 Desensitizes MC3T3-E1 Osteoblastic Cells to Estrogen Through Transcriptional Downregulation of Estrogen Receptor 1
- Immunohistochemical Study on the Distribution of Estrogen Receptor-alpha in the Hippocampus of the Normal Aged Rat