Immune Netw.
2014 Feb;14(1):54-65. 10.4110/in.2014.14.1.54.
Molecular Characterization of Neurally Differentiated Human Bone Marrow-derived Clonal Mesenchymal Stem Cells
- Affiliations
-
- 1Translational Research Center, Inha University School of Medicine, Incheon 400-712, Korea. sunuksong@inha.ac.kr, msjeon@inha.ac.kr
- 2Inha Research Institute for Medical Sciences of Biomedical Sciences, Inha University School of Medicine, Incheon 400-712, Korea.
- 3Drug Development Program, Department of Medicine, Inha University School of Medicine, Incheon 400-712, Korea.
- 4HomeoTherapy Co. Ltd., Incheon 400-711, Korea.
- KMID: 1508829
- DOI: http://doi.org/10.4110/in.2014.14.1.54
Abstract
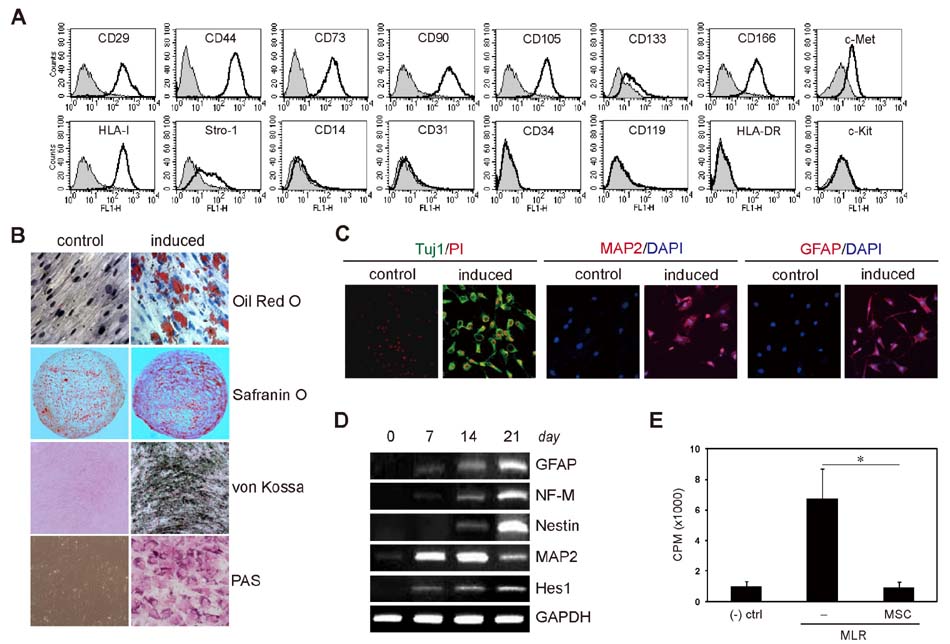
- Bone marrow-derived mesenchymal stem cells (MSCs) are multipotent, with the ability to differentiate into different cell types. Additionally, the immunomodulatory activity of MSCs can downregulate inflammatory responses. The use of MSCs to repair injured tissues and treat inflammation, including in neuroimmune diseases, has been extensively explored. Although MSCs have emerged as a promising resource for the treatment of neuroimmune diseases, attempts to define the molecular properties of MSCs have been limited by the heterogeneity of MSC populations. We recently developed a new method, the subfractionation culturing method, to isolate homogeneous human clonal MSCs (hcMSCs). The hcMSCs were able to differentiate into fat, cartilage, bone, neuroglia, and liver cell types. In this study, to better understand the properties of neurally differentiated MSCs, gene expression in highly homogeneous hcMSCs was analyzed. Neural differentiation of hcMSCs was induced for 14 days. Thereafter, RNA and genomic DNA was isolated and subjected to microarray analysis and DNA methylation array analysis, respectively. We correlated the transcriptome of hcMSCs during neural differentiation with the DNA methylation status. Here, we describe and discuss the gene expression profile of neurally differentiated hcMSCs. These findings will expand our understanding of the molecular properties of MSCs and contribute to the development of cell therapy for neuroimmune diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow; Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6:230–247.2. Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009; 1176:101–117.
Article3. Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012; 35:213–221.
Article4. Yoo HS, Yi T, Cho YK, Kim WC, Song SU, Jeon MS. Mesenchymal stem cell lines isolated by different isolation methods show variations in the regulation of graft-versus-host disease. Immune Netw. 2013; 13:133–140.
Article5. Anderson P, Souza-Moreira L, Morell M, Caro M, O'Valle F, Gonzalez-Rey E, Delgado M. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013; 62:1131–1141.
Article6. Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011; 140:998–1008.
Article7. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
Article8. Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, Valentine VG, Prockop DJ, Kolls JK. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005; 102:186–191.
Article9. Tao XR, Li WL, Su J, Jin CX, Wang XM, Li JX, Hu JK, Xiang ZH, Lau JT, Hu YP. Clonal mesenchymal stem cells derived from human bone marrow can differentiate into hepatocyte-like cells in injured livers of SCID mice. J Cell Biochem. 2009; 108:693–704.
Article10. Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002; 99:2199–2204.
Article11. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370.
Article12. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001; 282:148–152.
Article13. Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res. 2004; 77:192–204.
Article14. Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004; 77:174–191.
Article15. Mareschi K, Novara M, Rustichelli D, Ferrero I, Guido D, Carbone E, Medico E, Madon E, Vercelli A, Fagioli F. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Exp Hematol. 2006; 34:1563–1572.
Article16. Kim S, Honmou O, Kato K, Nonaka T, Houkin K, Hamada H, Kocsis JD. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006; 1123:27–33.
Article17. Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010; 16:203–209.
Article18. Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, Lavon I, Baniyash M, Lassmann H, Ben-Hur T. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 2007; 61:209–218.
Article19. Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008; 265:102–104.
Article20. Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996; 11:312–324.
Article21. Song SU, Kim CS, Yoon SP, Kim SK, Lee MH, Kang JS, Choi GS, Moon SH, Choi MS, Cho YK, Son BK. Variations of clonal marrow stem cell lines established from human bone marrow in surface epitopes, differentiation potential, gene expression, and cytokine secretion. Stem Cells Dev. 2008; 17:451–461.
Article22. Tondreau T, Dejeneffe M, Meuleman N, Stamatopoulos B, Delforge A, Martiat P, Bron D, Lagneaux L. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics. 2008; 9:166.
Article23. Shakhbazau AV, Goncharova NV, Kosmacheva SM, Kartel NA, Potanev MP. Plasticity of human msesnchymal stem cell phenotype and expression profile under neurogenic conditions. Bull Exp Biol Med. 2009; 147:513–516.
Article24. Yamaguchi S, Kuroda S, Kobayashi H, Shichinohe H, Yano S, Hida K, Shinpo K, Kikuchi S, Iwasaki Y. The effects of neuronal induction on gene expression profile in bone marrow stromal cells (BMSC)-a preliminary study using microarray analysis. Brain Res. 2006; 1087:15–27.
Article25. Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001; 293:1089–1093.
Article26. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003; 33:Suppl. 245–254.
Article27. Larsen F, Gundersen G, Lopez R, Prydz H. CpG islands as gene markers in the human genome. Genomics. 1992; 13:1095–1107.
Article28. Bird AP, Wolffe AP. Methylation-induced repression--belts, braces, and chromatin. Cell. 1999; 99:451–454.
Article29. Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006; 133:4451–4461.
Article30. Gulacsi AA, Anderson SA. Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat Neurosci. 2008; 11:1383–1391.
Article31. Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008; 86:281–296.
Article32. Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retroelements during adult neurogenesis. Nat Neurosci. 2009; 12:1097–1105.
Article33. Garcia-Morales C, Liu CH, Abu-Elmagd M, Hajihosseini MK, Wheeler GN. Frizzled-10 promotes sensory neuron development in Xenopus embryos. Dev Biol. 2009; 335:143–155.
Article34. Ardley HC, Robinson PA. E3 ubiquitin ligases. Essays Biochem. 2005; 41:15–30.
Article35. Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009; 78:399–434.
Article36. Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, Chanda SK, Batalov S, Joazeiro CA. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle's dynamics and signaling. PLoS One. 2008; 3:e1487.
Article37. Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009; 10:398–409.
Article38. Schwamborn JC, Muller M, Becker AH, Puschel AW. Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J. 2007; 26:1410–1422.
Article39. Bryan B, Cai Y, Wrighton K, Wu G, Feng XH, Liu M. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 2005; 579:1015–1019.
Article40. Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012; 20:14–20.
Article41. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009; 5:54–63.
Article42. Oh JY, Roddy GW, Choi H, Lee RH, Ylostalo JH, Rosa RH Jr, Prockop DJ. Antiinflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010; 107:16875–16880.
Article43. Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011; 118:330–338.
Article44. Dripps DJ, Brandhuber BJ, Thompson RC, Eisenberg SP. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem. 1991; 266:10331–10336.
Article45. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012; 11:633–652.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Characterization of multipotent mesenchymal stem cells isolated from adipose tissue and bone marrow in pigs
- Neural Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Applicability for Inner Ear Therapy
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Bone Marrow-Derived Mesenchymal Stem Cells for Regenerative Medicine