Immune Netw.
2014 Feb;14(1):38-44. 10.4110/in.2014.14.1.38.
Gut-residing Microbes Alter the Host Susceptibility to Autoantibody-mediated Arthritis
- Affiliations
-
- 1Department of Anatomy & Cell Biology, College of Medicine, Hanyang University, Seoul 133-791, Korea. jhyoun@hanyang.ac.kr
- 2Department of Internal Medicine, College of Medicine, Hanyang University, Seoul 133-791, Korea.
- 3Konkuk University Medical Center, Seoul 143-701, Korea.
- KMID: 1508827
- DOI: http://doi.org/10.4110/in.2014.14.1.38
Abstract
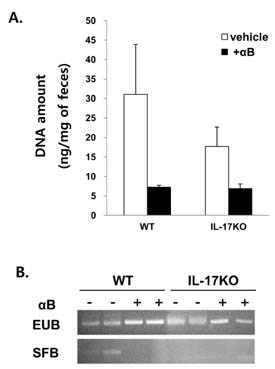
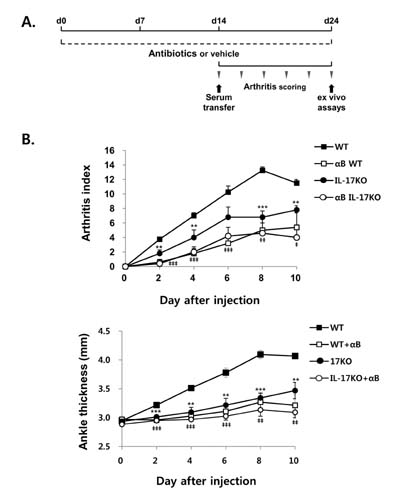
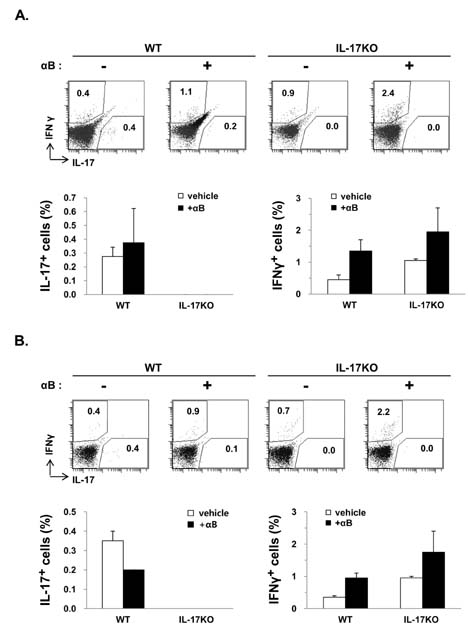
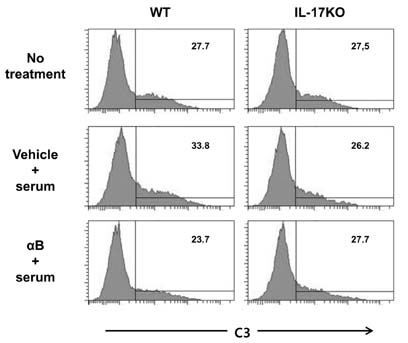
- K/BxN serum can transfer arthritis to normal mice owing to the abundant autoantibodies it contains, which trigger innate inflammatory cascades in joints. Little is known about whether gut-residing microbes affect host susceptibility to autoantibody-mediated arthritis. To address this, we fed C57BL/6 mice with water containing a mixture of antibiotics (ampicillin, vancomycin, neomycin, and metronidazol) for 2 weeks and then injected them with K/BxN serum. Antibiotic treatment significantly reduced the amount of bacterial genomic DNA isolated from fecal samples, in particular a gene encoding 16S ribosomal RNA derived from segmented filamentous bacteria. Arthritic signs, as indicated by the arthritic index and ankle thickness, were significantly attenuated in antibiotic-treated mice compared with untreated controls. Peyer's patches and mesenteric lymph nodes from antibiotic-treated mice contained fewer IL-17-expressing cells than those from untreated mice. Antibiotic treatment reduced serum C3 deposition in vitro via the alternative complement pathway. IL-17-/- congenic C57BL/6 mice were less susceptible to K/BxN serum-transferred arthritis than their wild-type littermates, but were still responsive to treatment with antibiotics. These results suggest that gut-residing microbes, including segmented filamentous bacteria, induce IL-17 production in GALT and complement activation via the alternative complement pathway, which cause the host to be more susceptible to autoantibody-mediated arthritis.
Keyword
MeSH Terms
-
Animals
Ankle
Anti-Bacterial Agents
Arthritis*
Autoantibodies
Bacteria
Complement Activation
Complement Pathway, Alternative
DNA
Genes, vif
Interleukin-17
Joints
Lymph Nodes
Mice
Neomycin
Peyer's Patches
RNA, Ribosomal, 16S
Vancomycin
Water
Anti-Bacterial Agents
Autoantibodies
DNA
Interleukin-17
Neomycin
RNA, Ribosomal, 16S
Vancomycin
Water
Figure
Cited by 1 articles
-
Interleukin 17-expressing Innate Synovial Cells Drive K/Bxn Serum-induced Arthritis
Wang Shik Cho, Eunkyeong Jang, Ho-Youn Kim, Jeehee Youn
Immune Netw. 2016;16(6):366-372. doi: 10.4110/in.2016.16.6.366.
Reference
-
1. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001; 358:903–911.
Article2. Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996; 87:811–822.
Article3. Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999; 10:451–461.
Article4. Ditzel HJ. The K/BxN mouse: a model of human inflammatory arthritis. Trends Mol Med. 2004; 10:40–45.
Article5. Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, Gravallese E, Mathis D, Benoist C. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002; 196:77–85.
Article6. Jang E, Cho SH, Park H, Paik DJ, Kim JM, Youn J. A positive feedback loop of IL-21 signaling provoked by homeostatic CD4+CD25- T cell expansion is essential for the development of arthritis in autoimmune K/BxN mice. J Immunol. 2009; 182:4649–4656.
Article7. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012; 336:1268–1273.
Article8. Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004; 4:478–485.
Article9. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008; 453:620–625.
Article10. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004; 303:1662–1665.
Article11. Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008; 180:559–568.
Article12. Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008; 4:337–349.
Article13. Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010; 32:815–827.
Article14. Jang E, Kim HR, Cho SH, Paik DJ, Kim JM, Lee SK, Youn J. Prevention of spontaneous arthritis by inhibiting homeostatic expansion of autoreactive CD4+ T cells in the K/BxN mouse model. Arthritis Rheum. 2006; 54:492–498.
Article15. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009; 139:485–498.
Article16. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009; 31:331–341.
Article17. Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010; 184:3336–3340.18. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010; 464:1371–1375.
Article19. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009; 27:485–517.
Article20. Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. Th17 cells can provide B cell help in autoantibody induced arthritis. J Autoimmun. 2011; 36:65–75.
Article21. Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci USA. 2010; 107:14292–14297.
Article22. Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001; 167:1601–1608.
Article23. Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009; 114:1005–1015.
Article24. Hashimoto M, Hirota K, Yoshitomi H, Maeda S, Teradaira S, Akizuki S, Prieto-Martin P, Nomura T, Sakaguchi N, Kohl J, Heyman B, Takahashi M, Fujita T, Mimori T, Sakaguchi S. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J Exp Med. 2010; 207:1135–1143.
Article25. Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010; 11:928–935.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status and Prospects of Intestinal Microbiome Studies
- Roles of intestinal epithelial cells in the maintenance of gut homeostasis
- B cells and autoantibody in RA pathogenesis
- The interplay between host immune cells and gut microbiota in chronic inflammatory diseases
- Gut microbiota affects brain development and behavior