Electrolyte Blood Press.
2011 Jun;9(1):7-15. 10.5049/EBP.2011.9.1.7.
The Role of Proximal Nephron in Cyclophosphamide-Induced Water Retention: Preliminary Data
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea. kimgh@hanyang.ac.kr
- 2Department of Veterinary, Chonnam National University College of Veterinary Medicine, Gwangju, Korea.
- KMID: 1497136
- DOI: http://doi.org/10.5049/EBP.2011.9.1.7
Abstract
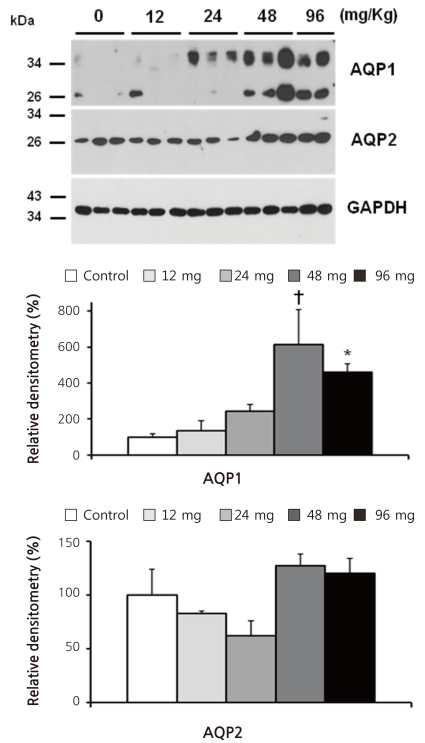
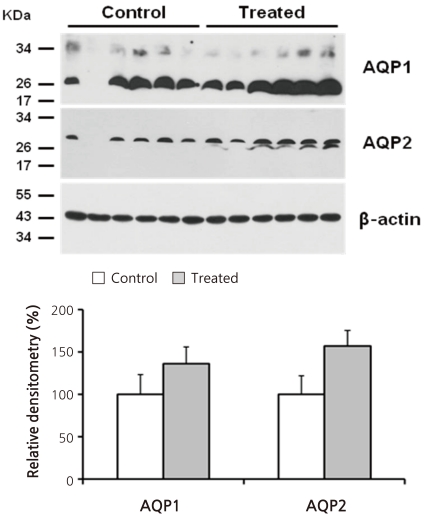
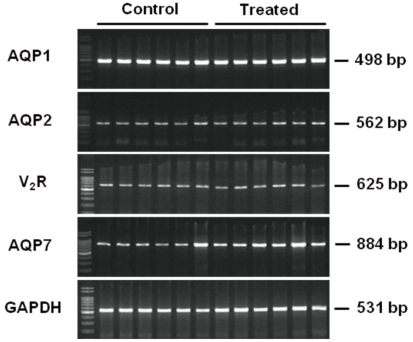
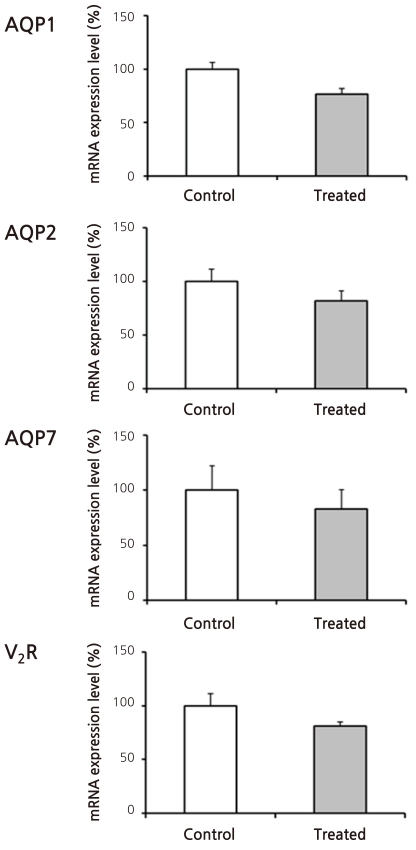
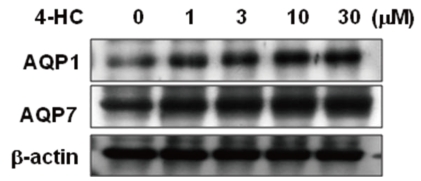
- Cyclophosphamide is clinically useful in treating malignancy and rheumatologic disease, but has limitations in that it induces hyponatremia. The mechanisms by which cyclophosphamide induces water retention in the kidney have yet to be identified. This study was undertaken to test the hypothesis that cyclophosphamide may produce water retention via the proximal nephron, where aquaporin-1 (AQP1) and aquaporin-7 (AQP7) water channels participate in water absorption. To test this hypothesis, we gave a single dose of intraperitoneal cyclophosphamide to male Sprague-Dawley rats and treated rabbit proximal tubule cells (PTCs) with 4-hydroperoxycyclophosphamide (4-HC), an active metabolite of cyclophosphamide. In the short-term 3-day rat study, AQP1 protein expression was significantly increased in the whole kidney homogenates by cyclophosphamide administration at 48 (614 +/- 194%, P < 0.005), and 96 (460 +/- 46%, P < 0.05) mg/kg BW compared with vehicle-treated controls. Plasma sodium concentration was significantly decreased (143 +/- 1 vs. 146 +/- 1 mEq/L, P < 0.05) by cyclophosphamide 100 mg/kg BW in the long-term 6-day rat study. When primary cultured rabbit PTCs were treated with 4-HC for 24 hours, the protein expressions of AQP1 and AQP7 were increased in a dose-dependent manner. Quantitative polymerase chain reaction revealed no significant changes in the mRNA levels of AQP1 and AQP7 from cyclophosphamide-treated rat renal cortices. From these preliminary data, we conclude that the proximal nephron may be involved in cyclophosphamide-induced water retention via AQP1 and AQP7 water channels. Further studies are required to demonstrate intracellular mechanisms that affect the expression of AQP proteins.
Keyword
MeSH Terms
Figure
Reference
-
1. Moses AM, Miller M. Drug-induced dilutional hyponatremia. N Engl J Med. 1974; 291:1234–1239. PMID: 4607502.
Article2. Steele TH, Serpick AA, Block JB. Antidiuretic response to cyclophosphamide in man. J Pharmacol Exp Ther. 1973; 185:245–253. PMID: 4703820.3. Koo TY, Bae SC, Park JS, et al. Water intoxication following low-dose intravenous cyclophosphamide. Electrolyte Blood Press. 2007; 5:50–54.
Article4. Lee YC, Park JS, Lee CH, et al. Hyponatraemia induced by low-dose intravenous pulse cyclophosphamide. Nephrol Dial Transplant. 2010; 25:1520–1524. PMID: 20007128.
Article5. Nielsen S, Agre P. The aquaporin family of water channels in kidney. Kidney Int. 1995; 48:1057–1068. PMID: 8569067.
Article6. Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A. 1993; 90:11663–11667. PMID: 8265605.
Article7. Lee YJ, Han HJ. Troglitazone ameliorates high glucose-induced EMT and dysfunction of SGLTs through PI3K/Akt, GSK-3{beta}, Snail1, and {beta}-catenin in renal proximal tubule cells. Am J Physiol Renal Physiol. 2010; 298:F1263–F1275.8. Bodo E, Tobin DJ, Kamenisch Y, et al. Dissecting the impact of chemotherapy on the human hair follicle: a pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy. Am J Pathol. 2007; 171:1153–1167. PMID: 17823286.9. Flowers JL, Ludeman SM, Gamcsik MP, et al. Evidence for a role of chloroethylaziridine in the cytotoxicity of cyclophosphamide. Cancer Chemother Pharmacol. 2000; 45:335–344. PMID: 10755323.
Article10. Anderson LW, Ludeman SM, Colvin OM, Grochow LB, Strong JM. Quantitation of 4-hydroxycyclophosphamide/aldophosphamide in whole blood. J Chromatogr B Biomed Appl. 1995; 667:247–257. PMID: 7663697.
Article11. Anderson LW, Chen TL, Colvin OM, et al. Cyclophosphamide and 4-Hydroxycyclophosphamide/aldophosphamide kinetics in patients receiving high-dose cyclophosphamide chemotherapy. Clin Cancer Res. 1996; 2:1481–1487. PMID: 9816324.12. Chen TL, Kennedy MJ, Anderson LW, et al. Nonlinear pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide/aldophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Drug Metab Dispos. 1997; 25:544–551. PMID: 9152592.13. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.
Article14. Lee CH, Kim S, Kang CM, Kim WY, Kim J, Kim GH. Altered expression of tight junction proteins in cyclosporine nephrotoxicity. Am J Nephrol. 2011; 33:7–16. PMID: 21124021.
Article15. Sonoda H, Yokota-Ikeda N, Oshikawa S, et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009; 297:F1006–F1016. PMID: 19640902.
Article16. Kishore BK, Mandon B, Oza NB, et al. Rat renal arcade segment expresses vasopressin-regulated water channel and vasopressin V2 receptor. J Clin Invest. 1996; 97:2763–2771. PMID: 8675687.
Article17. Nejsum LN, Elkjaer M, Hager H, Frokiaer J, Kwon TH, Nielsen S. Localization of aquaporin-7 in rat and mouse kidney using RT-PCR, immunoblotting, and immunocytochemistry. Biochem Biophys Res Commun. 2000; 277:164–170. PMID: 11027658.
Article18. Sarmiento JM, Ehrenfeld P, Anazco CC, et al. Differential distribution of the vasopressin V receptor along the rat nephron during renal ontogeny and maturation. Kidney Int. 2005; 68:487–496. PMID: 16014025.19. Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol. 1996; 271:F414–F422. PMID: 8770174.
Article20. Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis. 2008; 52:144–153. PMID: 18468754.
Article21. Bode U, Seif SM, Levine AS. Studies on the antidiuretic effect of cyclophosphamide: vasopressin release and sodium excretion. Med Pediatr Oncol. 1980; 8:295–303. PMID: 7464688.
Article22. Bressler RB, Huston DP. Water intoxication following moderate-dose intravenous cyclophosphamide. Arch Intern Med. 1985; 145:548–549. PMID: 3977522.
Article23. Campbell DM, Atkinson A, Gillis D, Sochett EB. Cyclophosphamide and water retention: mechanism revisited. J Pediatr Endocrinol Metab. 2000; 13:673–675. PMID: 10905395.
Article24. Kahn T. Reset osmostat and salt and water retention in the course of severe hyponatremia. Medicine (Baltimore). 2003; 82:170–176. PMID: 12792303.
Article25. DeFronzo RA, Braine H, Colvin M, Davis PJ. Water intoxication in man after cyclophosphamide therapy. Time course and relation to drug activation. Ann Intern Med. 1973; 78:861–869. PMID: 4713567.26. de Bragança AC, Moyses ZP, Magaldi AJ. Carbamazepine can induce kidney water absorption by increasing aquaporin 2 expression. Nephrol Dial Transplant. 2010; 25:3840–3845. PMID: 20525972.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Water Intoxication Following Low-Dose Intravenous Cyclophosphamide
- Renal Sodium Transporters and Water Channels
- Modulatory effect of water and/or food deprivation, and cyclophosphamide administration on immune response in mice
- Role of ¹â¸F-FDG PET-CT in Monitoring the Cyclophosphamide Induced Pulmonary Toxicity in Patients with Breast Cancer: 2 Case Reports
- Importance of Residual Water Permeability on the Excretion of Water during Water Diuresis in Rats