Electrolyte Blood Press.
2010 Jun;8(1):1-9. 10.5049/EBP.2010.8.1.1.
Importance of Residual Water Permeability on the Excretion of Water during Water Diuresis in Rats
- Affiliations
-
- 1Renal Divisions, St. Michael's Hospital, University of Toronto, Toronto, Ontario, Canada. mitchell.halperin@utoronto.ca
- 2Keenan Research Centre, Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, Ontario, Canada.
- KMID: 1462996
- DOI: http://doi.org/10.5049/EBP.2010.8.1.1
Abstract
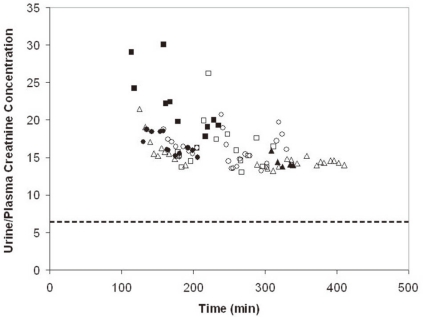
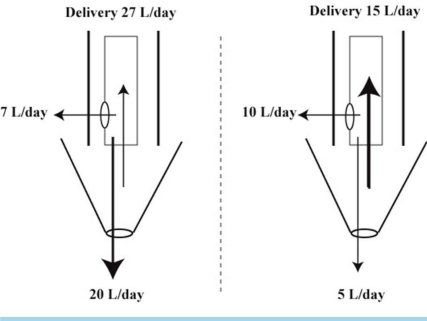
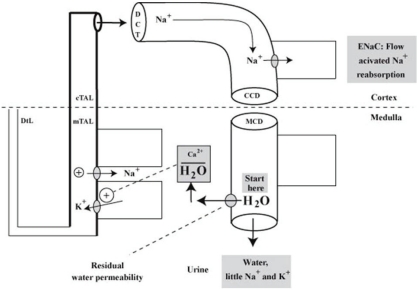
- When the concentration of sodium (Na+) in arterial plasma (P(Na)) declines sufficiently to inhibit the release of vasopressin, water will be excreted promptly when the vast majority of aquaporin 2 water channels (AQP2) have been removed from luminal membranes of late distal nephron segments. In this setting, the volume of filtrate delivered distally sets the upper limit on the magnitude of the water diuresis. Since there is an unknown volume of water reabsorbed in the late distal nephron, our objective was to provide a quantitative assessment of this parameter. Accordingly, rats were given a large oral water load, while minimizing non-osmotic stimuli for the release of vasopressin. The composition of plasma and urine were measured. The renal papilla was excised during the water diuresis to assess the osmotic driving force for water reabsorption in the inner medullary collecting duct. During water diuresis, the concentration of creatinine in the urine was 13-fold higher than in plasma, which implies that ~8% of filtered water was excreted. The papillary interstitial osmolality was 600 mOsm/L > the urine osmolality. Since 17% of filtered water is delivered to the earliest distal convoluted tubule micropuncture site, we conclude that half of the water delivered to the late distal nephron is reabsorbed downstream during water diuresis. The enormous osmotic driving force for the reabsorption of water in the inner medullary collecting duct may play a role in this reabsorption of water. Possible clinical implications are illustrated in the discussion of a case example.
Keyword
MeSH Terms
Figure
Reference
-
1. Robertson GL. Thirst and vasopressin. The Kidney. 2008. v.1:4th ed. New York: Raven press;p. 1123–1142.
Article2. Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002; 82:205–244. PMID: 11773613.3. Halperin ML, Kamel KS, Oh MS. Mechanisms to concentrate the urine: an opinion. Curr Opin Nephrol Hypertens. 2008; 17:416–422. PMID: 18660679.
Article4. Shafiee MA, Charest AF, Cheema-Dhadli S, et al. Defining conditions that lead to the retention of water: the importance of the arterial sodium concentration. Kidney Int. 2005; 67:613–621. PMID: 15673308.
Article5. Lankford SP, Chou CL, Terada Y, Wall SM, Wade JB, Knepper MA. Regulation of collecting duct water permeability independent of cAMP-mediated AVP response. Am J Physiol. 1991; 261:F554–F566. PMID: 1653534.
Article6. Halperin ML, Bichet DG, Oh MS. Integrative physiology of basal water permeability in the distal nephron: implications for the syndrome of inappropriate secretion of antidiuretic hormone. Clin Nephrol. 2001; 56:339–345. PMID: 11758003.7. Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007; 356:2064–2072. PMID: 17507705.8. Kamel KS, Cheema-Dhadli S, Shafiee MA, Halperin ML. Dogmas and controversies in the handling of nitrogenous wastes: excretion of nitrogenous wastes in human subjects. J Exp Biol. 2004; 207:1985–1991. PMID: 15143132.
Article9. Cheema-Dhadli S, Halperin ML. Relative rates of appearance of nitrogen and sulphur: implications for postprandial synthesis of proteins. Can J Physiol Pharmacol. 1993; 71:120–127. PMID: 8319135.
Article10. Halperin ML, Vinay P, Gougoux A, Pichette C, Jungas RL. Regulation of the maximum rate of renal ammoniagenesis in the acidotic dog. Am J Physiol. 1985; 248:F607–F615. PMID: 3985167.
Article11. Gottschalk CW. Micropuncture studies of tubular function in the mammalian kidney. Physiologist. 1961; 4:35–55.12. Walser M. Creatinine excretion as a measure of protein nutrition in adults of varying age. JPEN J Parenter Enteral Nutr. 1987; 11:73S–78S. PMID: 3312696.
Article13. Gottschalk CW, Mylle M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol. 1959; 196:927–936. PMID: 13637248.
Article14. Gottschalk CW. Osmotic Concentration and Dilution of the Urine. Am J Med. 1964; 36:670–685. PMID: 14141447.
Article15. Agre P, Preston GM, Smith BL, et al. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993; 265:F463–F476. PMID: 7694481.
Article16. Zhai XY, Fenton RA, Andreasen A, Thomsen JS, Christensen EI. Aquaporin-1 is not expressed in descending thin limbs of short-loop nephrons. J Am Soc Nephrol. 2007; 18:2937–2944. PMID: 17942963.
Article17. Halperin M, Oh M, Kamel K. Integrating effects of aquaporins, vasopressin, distal delivery of filtrate and residual water permeability on the magnitude of water diuresis. Nephron Physiol. 2010; 114:p11–p17. PMID: 20110734.
Article18. Schmidt-Nielsen B, Churchill M, Reinking LN. Occurrence of renal pelvic refluxes during rising urine flow rate in rats and hamsters. Kidney Int. 1980; 18:419–431. PMID: 7230608.
Article19. Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985; 312:283–289. PMID: 2981409.20. Quinton PM. Physiology of sweat secretion. Kidney Int Suppl. 1987; 21:S102–S108. PMID: 3306099.21. Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989; 20:537–563. PMID: 2654204.
Article22. Davison JM, Vallotton MB, Lindheimer MD. Plasma osmolality and urinary concentration and dilution during and after pregnancy: evidence that lateral recumbency inhibits maximal urinary concentrating ability. Br J Obstet Gynaecol. 1981; 88:472–479. PMID: 7236550.
Article23. Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980; 18:152–161. PMID: 7003196.24. Lindheimer MD, Barron WM, Davison JM. Osmoregulation of thirst and vasopressin release in pregnancy. Am J Physiol. 1989; 257:F159–F169. PMID: 2669525.
Article25. Schreiber M, Halperin M. The Paleolithic curriculum: figure it out (with the help of experts). Adv Physiol Educ. 1998; 275:S185–S194.
Article26. Davids MR, Edoute Y, Stock S, Halperin ML. Severe degree of hyperglycaemia: insights from integrative physiology. QJM. 2002; 95:113–124. PMID: 11861959.
Article27. Voicu L, Hare G, Mazer D, Cheema-Dhadli S, Halperin ML. Mechanisms to reduce hypoxic damage in the loop of Henle. J Am Soc Nephrol. 2009; 20:In press.28. Rossier BC, Canessa CM, Schild L, Horisberger JD. Epithelial sodium channels. Curr Opin Nephrol Hypertens. 1994; 3:487–496. PMID: 7804746.
Article29. Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol. 2006; 291:F923–F931. PMID: 16849691.
Article30. Thaler SM, Teitelbaum I, Berl T. "Beer potomania" in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998; 31:1028–1031. PMID: 9631849.
Article31. Oh MS, Carroll HJ, Roy A, et al. Chronic hyponatremia in the absence of ADH: possible role of decreased delivery of filtrate. J Am Soc Nephrol. 1997; 8:108A.32. Halperin ML, Davids MR, Kamel KS. Interpretation of urinary electrolyte and acid-base parameters. Brenner and Rectors The Kidney. 2004. v.1:7th ed. Philadelphia: WB Saunders;p. 1151–1181.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Free Water Clearance: A Clinical marker of Patients with Obstructive Uropathy
- Pathogenesis of Postobstructive Diuresis: Role of Aquaporin Water Channels, Sodium Transporters and Natriuretic Peptide System in the Kidney in Rats
- Mineral Water Investigation on 10 Area in Seoul
- Changes in renal brush-border sodium-dependent transport systems in gentamicin-treated rats
- Urinary Concentration Defect and Renal Glycosuria in Cyclosporine-treated Rats