J Korean Ophthalmol Soc.
2008 Jul;49(7):1135-1145. 10.3341/jkos.2007.49.7.1135.
The Role of Endothelial Progenital Cells and Fibrin on Vascularization and Stability in Orbital Implant
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Inje University, Pusan, Korea. jwsyhyo@yahoo.co.kr
- 2Department of Microbiology, College of Medicine, Inje University, Pusan, Korea.
- 3Department of Pathology, College of Medicine, Inje University, Pusan, Korea.
- KMID: 1476969
- DOI: http://doi.org/10.3341/jkos.2007.49.7.1135
Abstract
-
PURPOSE: The effects of endothelial progenitor cells (EPCs) and fibrin on fibrovascular growth into porous polyethylene orbital implants (Medpor(R) sheet) were investigated using stem cells.
METHODS
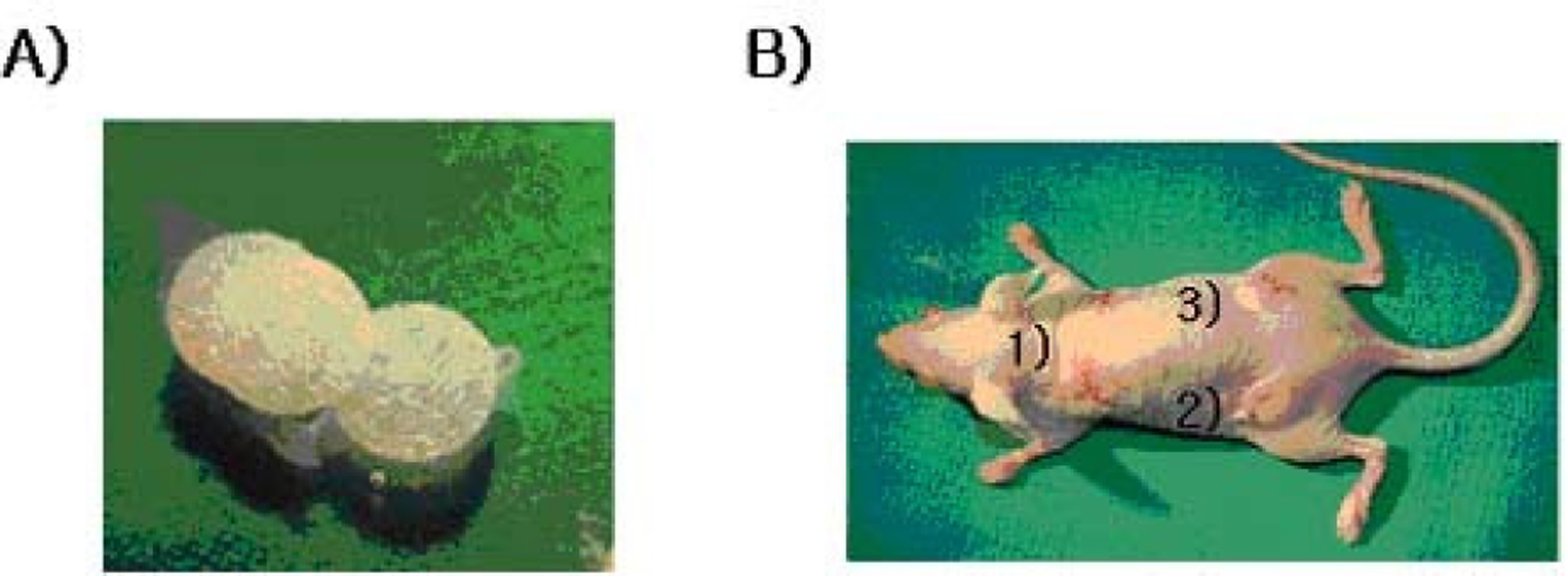
EPCs were separated from human adipose fat tissue for culture. Fluorescence-activated cell sorting (FACS) was used to identify the phenotype and to analyze the purity of EPCs cultivated from human adipose tissue. Processed Medpor(R) sheets were inserted in each quadrant of the subcutaneous fat layer under the dorsal surface of 20 anesthetized athymic nude mice, using sterile methods. Medpor(R) sheets processed with endothelial progenitor cells and fibrin were inserted into the two top quadrants, a Medpor(R) sheet processed with fibrin was inserted in the lower right quadrant, and an unprocessed Medpor(R) sheet was inserted in the lower left quadrant of each mouse. The mice were sacrificed on the seventh day. The adhesiveness and blood vessel formation were quantified by weight and the number of blood cells within the Medpor(R) sheets. Hematoxylin and eosin (H&E) and toluidine blue stains were used to analyze fibrovascular and cell growth within the Medpor(R) sheets.
RESULTS
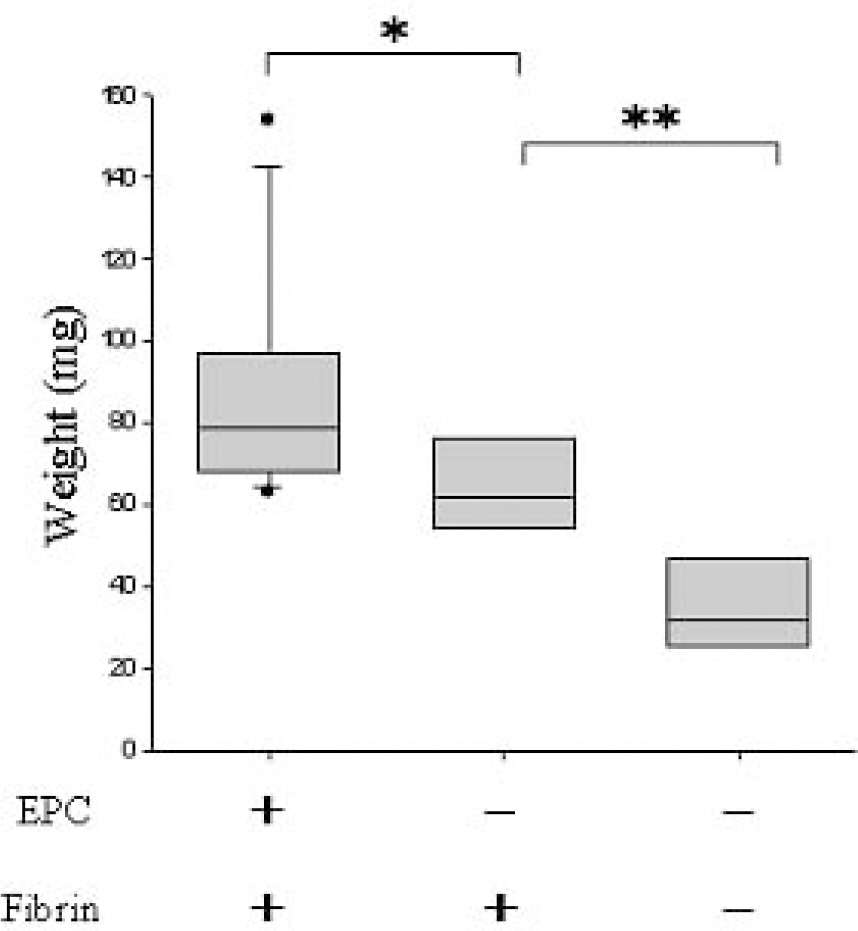
The sheets processed with EPCs and fibrin were heavier and contained more white and red blood cells (p<0.001) than the other sheets. The sheets processed with fibrin alone were heavier (p<0.01) and contained more blood cells (p<0.001) than the unprocessed sheets. The degree of vessel formation and tissue adhesiveness was greatest in the group of Medpor(R) sheets processed with EPCs and fibrin. The sheets processed with fibrin only had greater tissue adhesiveness and fibrovascular proliferation than the unprocessed Medpor(R) sheets.
CONCLUSIONS
Endothelial progenitor cells and fibrin applied to Medpor(R) sheets improve fibrovascular proliferation and tissue adhesiveness. When both are applied together, a synergistic effect is seen.
MeSH Terms
-
Adhesiveness
Adipose Tissue
Animals
Blood Cells
Blood Vessels
Coloring Agents
Eosine Yellowish-(YS)
Erythrocytes
European Continental Ancestry Group
Fibrin
Flow Cytometry
Glycosaminoglycans
Hematoxylin
Humans
Mice
Mice, Nude
Orbit
Orbital Implants
Phenotype
Polyethylene
Stem Cells
Subcutaneous Fat
Tolonium Chloride
Coloring Agents
Eosine Yellowish-(YS)
Fibrin
Glycosaminoglycans
Hematoxylin
Polyethylene
Tolonium Chloride
Figure
Reference
-
References
1. Choi JC, Iwamoto MA, Bstandig S. . Medpor® motility coupling post: a rabbit model. Ophthal Plast Reconstr Surg. 1999; 15:190–201.2. Mawn LA, Jordan DR, Gilberg S. Scanning electron microscopic examination of porous orbital implants. Can J Ophthalmol. 1998; 33:203–9.3. Rubin PA, Nicaeus TE, Warner MA, Remulla H. Effect of sucralfate and basic fibroblast growth factor on fibrovascular ingrowth into hydroxyapatite and porous polyethylene alloplastic implants using a novel rabbit model. Ophthal Plast Reconstr Surg. 1997; 13:8–17.
Article4. Remulla HD, Rubin PA, Shore JW. . Complications of porous spherical orbital implants. Ophthalmology. 1995; 102:586–93.
Article5. Rubin PA, Popham JK, Bilyk JR, Shore JW. Comparison of fibrovascular ingrowth into hydroxyapatite and porous polyethylene orbital implants. Ophthal Plast Reconstr Surg. 1994; 10:96–103.
Article6. Karesh JW, Dresner SC. High-density porous polyethylene (Medpor®) as a successful anophthalmic socket implant. Ophthalmology. 1994; 101:1688–95.7. Jordan DR, Brownstein S, Jolly SS. Abscessed hydroxyapatite orbital implants. Ophthalmology. 1996; 103:1784–7.
Article8. Karcioglu ZA. Actinomyces infection in porous polyethylene orbital implant. Graefes Arch Clin Exp Ophthalmol. 1997; 235:448–51.
Article9. Soparkar CN, Wong JF, Patrinely JR. Appling D. Epidermal and fibroblast growth factors enhance fibrovascular integration of porous polyethylene implants. Ophthal Plast Reconstr Surg. 2000; 16:337–40.10. Kader KN, Moore LR, Saul JM. . Isolation and Purification of Canine Adipose Microvascular Endothelial cells. Microvas Res. 2001; 61:220–6.
Article11. Kenny D, Coughlan MG, Pagel PS. . Transforming growth factor beta 1 preserves endothelial function after multiple brief coronary artery occlusions and reperfusion. Am Heart J. 1994; 127:1456–61.12. Rinsch C, Quinodoz P, Pittet B. . Delivery of FGF-2 but not VEGF by encapsulated genetically engineered myoblasts improves survival and vascularization in a model of acute skin flap ischemia. Gene Ther. 2001; 8:523–33.
Article13. Rashid MA, Akita S, Razzaque MS. . Coadministration of basic fibroblast growth factor and sucrose octasulfate (sucralfate) facilitates the rat dorsal flap survival and viability. Plast Reconstr Surg. 1999; 103:941–8.
Article14. Li QF, Reis ED, Zhang WX. . Accelerated flap prefabrication with vascular endothelial growth factor. J Reconstr Microsurg. 2000; 16:45–9.
Article15. Taub PJ, Marmur JD, Zhang WX. . Effect of time on the viability of ischemic skin flaps treated with vascular endothelial growth factor (VEGF) cDNA. J Reconstr Microsurg. 1998; 14:387–90.
Article16. Kryger Z, Dogan T, Zhang F. . VEGF administration following ischemia on survival of the gracilis muscle flap in the rat. Ann Plast Surg. 1999; 43:172–8.17. Kryger Z, Zhang F, Dogan T. . The effects of VEGF on survival of a random flap in the rat: Examination of various routes of administration. Br J Plast Surg. 2000; 53:234–9.
Article18. Banbury J, Siemionow M, Porvasnik S. . Improved perfusion after subcritical ischemia in muscle flaps treated with vascular endothelial growth factor. Plast Reconstr Surg. 2000; 106:1541–6.
Article19. Park Sh, Tepper OM, Galiano RD. . Selective Recruitment of Endothelial Progenitor Cells to Ischemic Tissues with Increased Neovascularization. Plast Reconstr Surg. 2004; 113:284–93.
Article20. Roberts AB, Sporn MB, Assoian RK. . Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986; 83:4167–71.
Article21. Buettner H, Bartley GB. Tissue breakdown and exposure associated with orbital hydroxyapatite implants. Am J Ophthalmol. 1992; 113:669–73.
Article22. Ainbinder DJ, Haik BG, Tellado M. Hydroxyapatite orbital implant abscess: histopathologic correlation of an infected implant following evisceration. Ophthal Plast Reconstr Surg. 1994; 10:267–70.23. Beaver HA, Patrinely JR, Holds JB, Soper MP. Periocular autografts in socket reconstruction. Ophthalmology. 1996; 103:149–8-502.
Article24. Perry AC. Integrated orbital implant. Adv Ophthalmic Plast Reconstr Surg. 1990; 8:75–81.25. Goldberg RA, Dresner SC, Braslow RA. . Animal model of porous polyethylene orbital implants. Ophthal Plast Reconstr Surg. 1994; 10:104–9.
Article26. Bigham WJ, Stanley p, Cahill JM Jr. . Fibrovascular ingrowth in porous ocular implants: the effect of material composition, porosity, growth factors and coatings. Ophthal Plast Reconstr Surg. 1999; 15:317–25.27. Asahara T, Takahashi T, Masuda H. . VEGF contributes to postnatal neovascularization by mobilizing bone marrow- derived endothelial progenital cells. Embo J. 1999; 18:3964–72.28. Kalka C, Masuda H, Takahashi T. . Transplantation of ex vivo expanded endothelial progenital cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000; 97:3422–7.29. Rosenthal AR, Harbury C, Egbert PR, Rubenstein E.Use of a platelet-fibrinogen-thrombin mixture as a corneal adhesive: experiments with sutureless lamellar keratoplasty in the rabbit. Invest Ophthalmol. 1975; 14:872.30. Tiwari A, Hamilton G, Seifalian AM. . Isolation of endothelial progenital cells and their progenitor cells from human perpheral blood. J Vasc Surg. 2002; 35:827.31. Schlag G, Redl H, Turnher M, Dinges HP. Importance of fibrin in wound repair. Rev Laryngol Otol Rhinol (Bord). 1987; 108:5–6.32. Clark RA. Fibrin and wound healing. Ann NY Acad Sci. 2001; 936:355–7.
Article33. Greer DJ, Andreadis ST. A novel role of fibrin in epidermal healing: plasminogen-mediated migration and selective detachment of differentiated keratinocytes. J Invest Dermatol. 2003; 121:1210–6.34. Pospisil CH, Stafford AR, Fredenburgh JC, Weitz JI. Evidence that both exosites on thrombin participate in its high affinity interaction with fibrin. J Biol Chem. 2003; 278:21584–91.
Article35. Sahni A, Odrljin T, Francis CW. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J Biol Chem. 1998; 273:7554–9.
Article36. Clark RA. Fibrin Is a Many Splendored Thing. J Invest Dermatol. 2003; 121:111–2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Vascular Endothelial Growth Factor on Vascularization in Porous Polyethylene Orbital Implant (Medpor(R))
- Evaluation of VAscularization of Hydroxyapatite Orbital Implants after Mokified Implantation Procedure
- Experimental study on vascularization of autogenous bone graft using fibrin sealant
- Histopathologic Comparison of Vascularization between Dacron and Donor Sclera as Wrapping Material in Hydroxyapatite Implantation
- In Vivo Observation of Endothelial Cell-Assisted Vascularization in Pancreatic Cancer Xenograft Engineering