Immune Netw.
2009 Oct;9(5):192-202. 10.4110/in.2009.9.5.192.
Nitric Oxide Synthesis is Modulated by 1,25-Dihydroxyvitamin D3 and Interferon-gamma in Human Macrophages after Mycobacterial Infection
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chungnam National University, Daejeon 301-747, Korea. hayoungj@cnu.ac.kr
- 2Infection Signaling Network Research Center, College of Medicine, Chungnam National University, Daejeon 301-747, Korea.
- 3Department of Internal Medicine, College of Medicine, Konyang University, Nonsan 320-711, Korea.
- KMID: 1474580
- DOI: http://doi.org/10.4110/in.2009.9.5.192
Abstract
- BACKGROUND
Little information is available the role of Nitric Oxide (NO) in host defenses during human tuberculosis (TB) infection. We investigated the modulating factor(s) affecting NO synthase (iNOS) induction in human macrophages.
METHODS
Both iNOS mRNA and protein that regulate the growth of mycobacteria were determined using reverase transcriptase-polymerase chain reaction and western blot analysis. The upstream signaling pathways were further investigated using iNOS specific inhibitors.
RESULTS
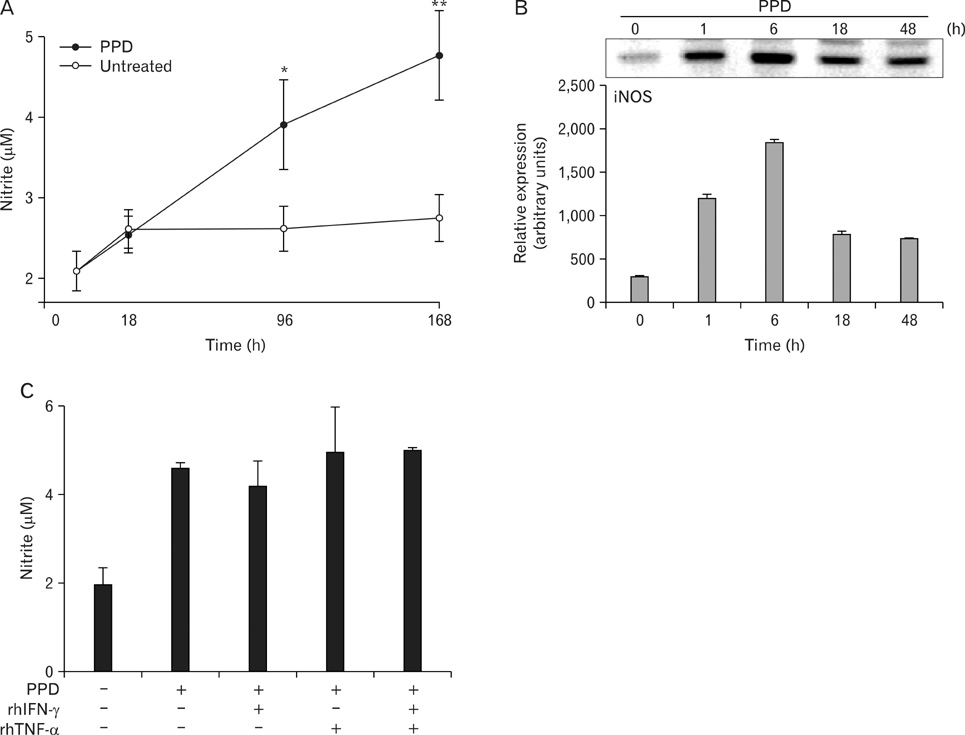
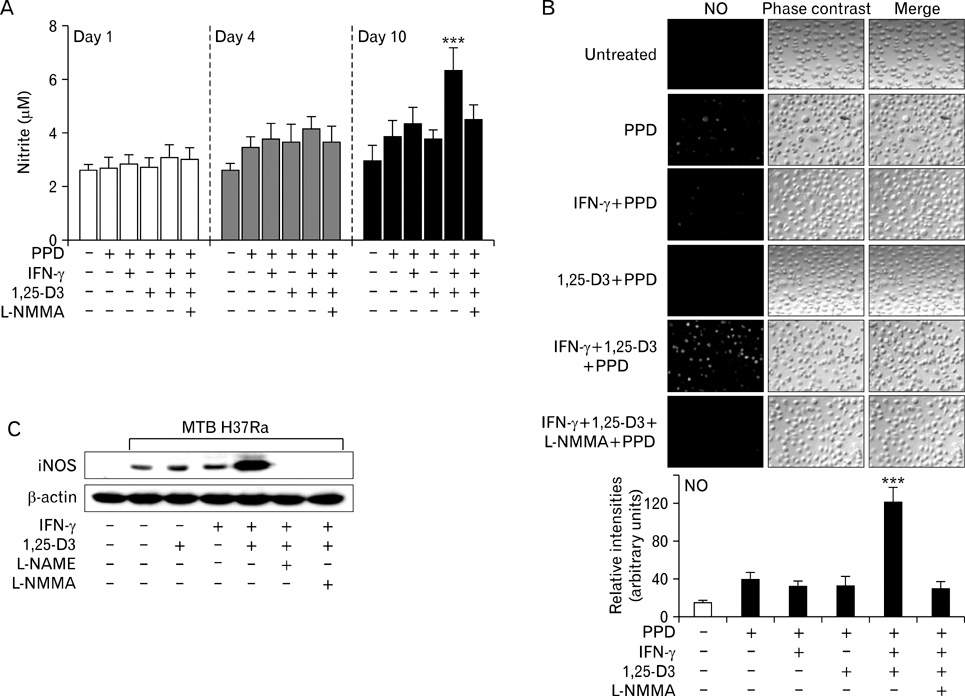
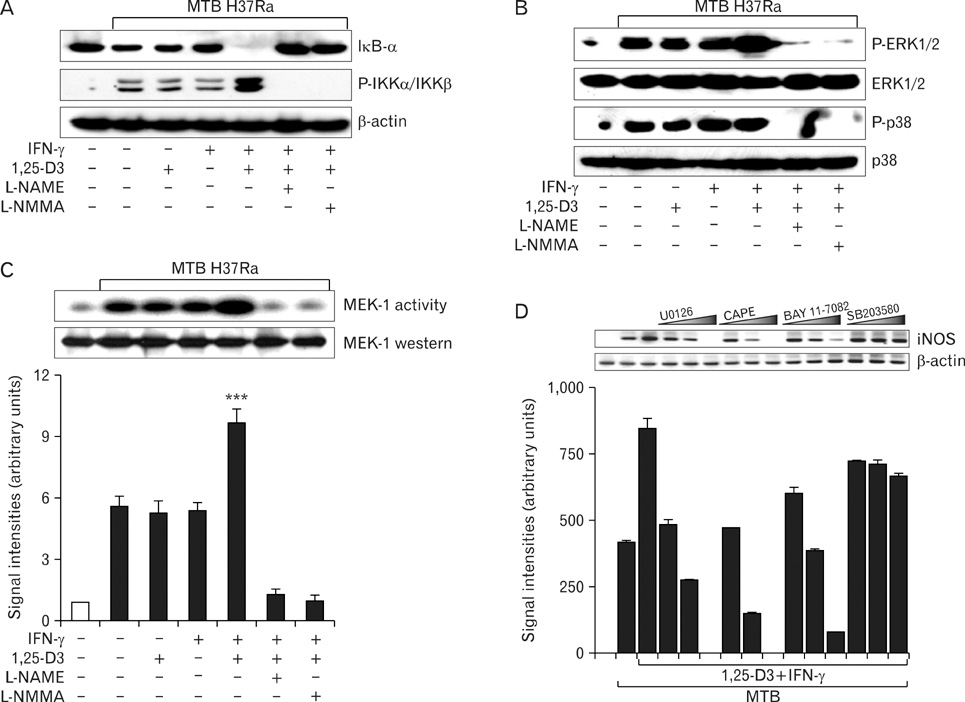
Here we show that combined treatment with 1,25-dihydroxyvitamin D3 (1,25-D3) and Interferon (IFN)-gamma synergistically enhanced NO synthesis and iNOS expression induced by Mycobacterium tuberculosis (MTB) or by its purified protein derivatives in human monocyte-derived macrophages. Both the nuclear factor-kappaB and MEK1-ERK1/2 pathways were indispensable in the induction of iNOS expression, as shown in toll like receptor 2 stimulation. Further, the combined treatment with 1,25-D3 and IFN-gamma was more potent than either agent alone in the inhibition of intracellular MTB growth. Notably, this enhanced effect was not explained by increased expression of cathelicidin, a known antimycobacterial effector of 1,25-D3.
CONCLUSION
These data support a key role of NO in host defenses against TB and identify novel modulating factors for iNOS induction in human macrophages.
Keyword
MeSH Terms
-
Antimicrobial Cationic Peptides
Blotting, Western
Calcitriol
Humans
Interferon-gamma
Interferons
Macrophages
Mycobacterium tuberculosis
Nitric Oxide
Nitric Oxide Synthase
RNA, Messenger
Toll-Like Receptor 2
Tuberculosis
Antimicrobial Cationic Peptides
Calcitriol
Interferon-gamma
Interferons
Nitric Oxide
Nitric Oxide Synthase
RNA, Messenger
Toll-Like Receptor 2
Figure
Reference
-
1. Tomioka H. Prospects for development of new antimycobacterial drugs. J Infect Chemother. 2000. 6:8–20.
Article2. Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992. 175:1111–1122.
Article3. Flynn JL, Scanga CA, Tanaka KE, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998. 160:1796–1803.4. Lon R, Light B, Talbot JA. Mycobacteriocidal action of exogenous nitric oxide. Antimicrob Agents Chemother. 1999. 43:403–405.
Article5. Yu K, Mitchell C, Xing Y, Magliozzo RS, Bloom BR, Chan J. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber Lung Dis. 1999. 79:191–198.
Article6. Nicholson S, Bonecini-Almeida Mad G, Lapa e Silva JR, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho JR. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996. 183:2293–2302.
Article7. Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998. 11:809–815.
Article8. Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, Toossi Z. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber Lung Dis. 1997. 78:247–255.
Article9. Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998. 66:5314–5321.
Article10. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006. 311:1770–1773.
Article11. Nathan C. Role of iNOS in human host defense. Science. 2006. 312:1874–1875.
Article12. Yang CS, Lee JS, Song CH, Hur GM, Lee SJ, Tanaka S, Akira S, Paik TH, Jo EK. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell Microbiol. 2007. 9:382–389.
Article13. Madrigal JL, Russo CD, Gavrilyuk V, Feinstein DL. Effects of noradrenaline on neuronal NOS2 expression and viability. Antioxid Redox Signal. 2006. 8:885–892.
Article14. Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1998. 141:2407–2412.15. Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS, Jo EK. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009. 182:3696–3705.
Article16. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997. 15:323–350.
Article17. Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993. 167:1358–1363.
Article18. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998. 16:225–260.
Article19. Jaramillo M, Gowda DC, Radzioch D, Olivier M. Hemozoin increases IFN-gamma-inducible macrophage nitric oxide generation through extracellular signal-regulated kinase- and NF-kappa B-dependent pathways. J Immunol. 2003. 171:4243–4253.
Article20. Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappaB. Proc Natl Acad Sci USA. 1996. 93:9090–9095.
Article21. Sugawara I, Yamada H, Li C, Mizuno S, Takeuchi O, Akira S. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003. 47:327–336.
Article22. Chan ED, Winston BW, Uh ST, Wynes MW, Rose DM, Riches DW. Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-γ and TNF-α in mouse macrophages. J Immunol. 1999. 162:415–422.23. Denis M. Tumor necrosis factor and granulocyte macrophage colony stimulating factor stimulate human macrophage to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991. 9:380–387.24. Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, Haney AF, Granger DL. Human mononuclear phagocytes inducible nitric oxide synthase (NOS): analysis if iNOS mRNA, iNOS protein, bioprotein and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995. 86:1184–1195.
Article25. Padgett EL, Pruett SB. Evaluation of nitrite production by human monocyte derived macrophages. Biochem Biophys Res Commun. 1992. 186:775–781.26. Sable SB, Goyal D, Verma I, Behera D, Khuller GK. Lung and blood mononuclear cell responses of TB patients to mycobacterial proteins. Eur Respir J. 2007. 29:337–346.
Article27. Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001. 291:1544–1547.
Article28. Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun. 1997. 65:3644–3647.
Article29. Bose M, Farnia P, Sharma S, Chattopadhya D, Saha K. Nitric oxide dependent killing of Mycobacterium tuberculosis by human mononuclear phagocytes from patients with active tuberculosis. Int J Immunopathol Pharmacol. 1999. 12:69–79.30. Jaramillo M, Naccache PH, Olivier M. Monosodium urate crystals synergize with IFN-gamma to generate macrophage nitric oxide: involvement of extracellular signal-regulated kinase 1/2 and NF-kappa B. J Immunol. 2004. 172:5734–5742.
Article31. Blanchette J, Jaramillo M, Olivier M. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology. 2003. 108:513–522.
Article32. Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002. 14:879–897.
Article33. Manolagas SC, Hustmyer FG, Yu XP. Immunomodulating properties of 1,25-dihydroxyvitamin D3. Kidney Int Suppl. 1990. 29:S9–S16.34. Remer KA, Brcic M, Sauter KS, Jungi TW. Human monocytoid cells as a model to study Toll-like receptor-mediated activation. J Immunol Methods. 2006. 313:1–10.
Article35. Davies PD. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle. 1985. 66:301–306.
Article36. Roy S, Frosham A, Saha B, Hazra SK, Mascie-Taylor CG, Hill AV. Association of vitamin D receptor genotype with leprosy type. J Infect Dis. 1999. 179:187–191.
Article37. Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000. 355:618–621.
Article38. Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987. 55:2945–2950.
Article39. Rook GA, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986. 57:159–163.40. Adams JS, Modlin RL, Diz MM, Barnes PF. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J Clin Endocrinol Metab. 1989. 69:457–460.
Article41. Dusso AS, Kamimura S, Gallieni M, Zhong M, Negrea L, Shapiro S, Slatopolsky E. Gamma-Interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab. 1997. 82:2222–2232.
Article42. Kikuchi H, Iizuka R, Sugiyama S, Gon G, Mori H, Arai M, Mizumoto K, Imajoh-Ohmi S. Monocytic differentiation modulates apoptotic response to cytotoxic anti-Fas antibody and tumor necrosis factor alpha in human monoblast U937 cells. J Leukoc Biol. 1996. 60:778–783.
Article43. Jourd'heuil D, Mills L, Miles AM, Grisham MB. Effect of nitric oxide on hemoprotein-catalyzed oxidative reactions. Nitric Oxide. 1998. 2:37–44.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nitric Oxide Synthesis in Murine Peritoneal Macrophages by Antithrombin III
- The Role of Nitric Oxide in Mycobacterial Infections
- Teat Shock Response Ingibits IFN-gamma Plus LPS - Induced NO Synthase Expression in Murine Peritoneal Macrophages
- Nitric Oxide Production in Mouse's Microglial Cells by Human Chorionic Gonadotropin
- Phosphatidylinositol-3-kinase Inhibitor Enhances Nitric Oxide Synthesis and Apoptosis in LPS-Stimulated Macrophages