Anat Cell Biol.
2010 Dec;43(4):325-331. 10.5115/acb.2010.43.4.325.
Direct protection of cultured neurons from ischemia-like injury by minocycline
- Affiliations
-
- 1Department of Anesthesia, Stanford University School of Medicine, Stanford, CA 94305, USA.
- 2Department of Neurosurgery, Stanford University School of Medicine, Stanford, CA 94305, USA.
- 3Department of Neurology, University of California, San Francisco and the San Francisco Veterans Affairs Medical Center, San Francisco, CA 94121, USA. yenari@alum.mit.edu
- KMID: 1455347
- DOI: http://doi.org/10.5115/acb.2010.43.4.325
Abstract
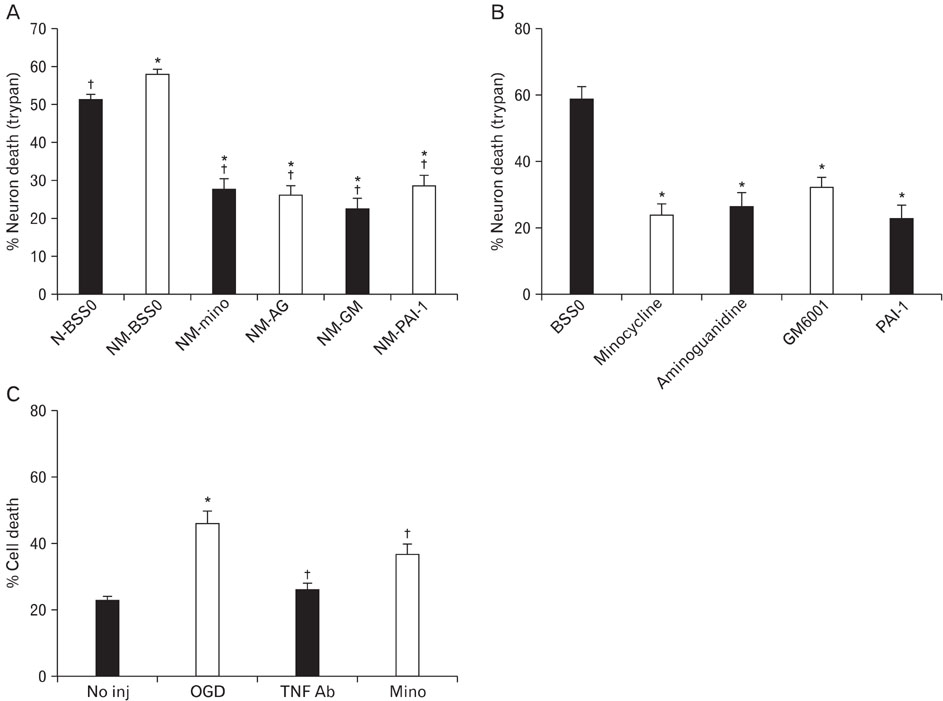
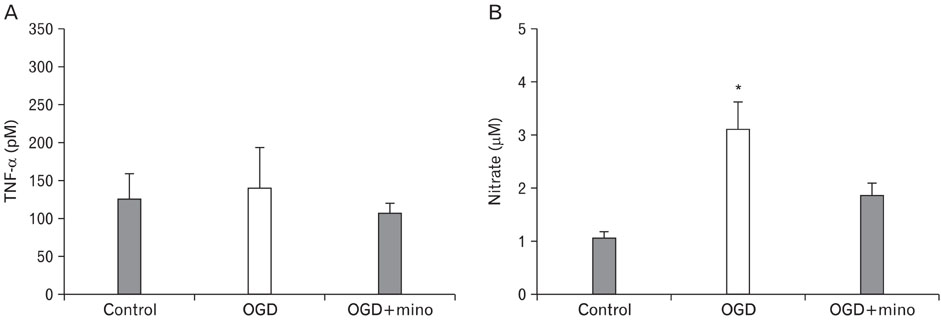
- Minocycline, a tetracycline antibiotic, is now known to protect cells via an anti-inflammatory mechanism. We further explored this effect using an in vitro model of ischemia-like injury to neurons. Coculturing neurons with microglia, the brain's resident immune cell, modestly increased cell death due to oxygen and glucose deprivation (OGD), compared to neurons alone. Treatment of cocultures with minocycline decreased cell death to a level significantly lower than that of neurons alone. Treatment of cocultures with minocycline or inhibitors of various immune mediators, also led to decreased cell death. Importantly, treatment of neuron cultures without added microglia with these same inhibitors of tissue plasminogen activator, matrix metalloproteinases, TNF-alpha and inducible nitric oxide synthase as well as minocycline also led to decreased cell death. Thus, anti-inflammatory treatments appear to be directly protective of neurons from in vitro ischemia.
Keyword
MeSH Terms
-
Cell Death
Coculture Techniques
Glucose
Ischemia
Matrix Metalloproteinases
Microglia
Minocycline
Neurons
Nitric Oxide Synthase Type II
Oxygen
Tetracycline
Tissue Plasminogen Activator
Tumor Necrosis Factor-alpha
Glucose
Matrix Metalloproteinases
Minocycline
Nitric Oxide Synthase Type II
Oxygen
Tetracycline
Tissue Plasminogen Activator
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990. 27:229–237.2. Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M. Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis. 2004. 17:359–366.3. Clark WM, Calcagno FA, Gabler WL, Smith JR, Coull BM. Reduction of central nervous system reperfusion injury in rabbits using doxycycline treatment. Stroke. 1994. 25:1411–1415.4. Corsani L, Bizzoco E, Pedata F, Gianfriddo M, Faussone-Pellegrini MS, Vannucchi MG. Inducible nitric oxide synthase appears and is co-expressed with the neuronal isoform in interneurons of the rat hippocampus after transient ischemia induced by middle cerebral artery occlusion. Exp Neurol. 2008. 211:433–440.5. Dugan LL, Bruno VM, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: glia exacerbate AMPA neurotoxicity. J Neurosci. 1995. 15:4545–4555.6. Flavin MP, Coughlin K, Ho LT. Soluble macrophage factors trigger apoptosis in cultured hippocampal neurons. Neuroscience. 1997. 80:437–448.7. Flavin MP, Ho LT. Propentofylline protects neurons in culture from death triggered by macrophage or microglial secretory products. J Neurosci Res. 1999. 56:54–59.8. Flavin MP, Zhao G. Tissue plasminogen activator protects hippocampal neurons from oxygen-glucose deprivation injury. J Neurosci Res. 2001. 63:388–394.9. Flavin MP, Zhao G, Ho LT. Microglial tissue plasminogen activator (tPA) triggers neuronal apoptosis in vitro. Glia. 2000. 29:347–354.10. Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007. 6:1045–1053.11. Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. 2002. 22:3921–3928.12. Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007. 69:1404–1410.13. Lee JE, Yenari MA, Sun GH, et al. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001. 170:129–139.14. Liu T, Clark RK, McDonnell PC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994. 25:1481–1488.15. Siao CJ, Fernandez SR, Tsirka SE. Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury. J Neurosci. 2003. 23:3234–3242.16. Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002. 513:87–113.17. Tang XN, Wang Q, Koike MA, et al. Monitoring the protective effects of minocycline treatment with radiolabeled annexin V in an experimental model of focal cerebral ischemia. J Nucl Med. 2007. 48:1822–1828.18. Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001. 166:7527–7533.19. Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007. 184:53–68.20. Wang X, Zhu S, Drozda M, et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci U S A. 2003. 100:10483–10487.21. Yenari MA, Giffard RG. Ischemic vulnerability of primary murine microglial cultures. Neurosci Lett. 2001. 298:5–8.22. Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006. 37:1087–1093.23. Ying W, Han SK, Miller JW, Swanson RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem. 1999. 73:1549–1556.24. Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998. 95:15769–15774.25. Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999. 96:13496–13500.26. Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008. 28:53–63.27. Zhu S, Stavrovskaya IG, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002. 417:74–78.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanism of Hypoxia-Induced Cytotoxicity in Cultured Rat Retinal Neurons
- Motor Recovery Effect of Minocycline in Spinal Cord Injured Rats
- Minocycline-Induced Autoimmune Hepatitis: A Rare But Important Cause of Drug-Induced Autoimmune Hepatitis
- Expression and Activity of the Na-K ATPase in Ischemic Injury of Primary Cultured Astrocytes
- The Neuroprotective Effect of Kefir on Spinal Cord Ischemia/Reperfusion Injury in Rats