Korean J Physiol Pharmacol.
2011 Aug;15(4):211-216. 10.4196/kjpp.2011.15.4.211.
Thrombin-induced Migration and Matrix Metalloproteinase-9 Expression Are Regulated by MAPK and PI3K Pathways in C6 Glioma Cells
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Kangwon National University, Kangwon 200-701, Korea. ksslsy@kangwon.ac.kr
- 2Department of System Immunology, College of Biomedical Science, Kangwon National University, Kangwon 200-701, Korea.
- KMID: 1448926
- DOI: http://doi.org/10.4196/kjpp.2011.15.4.211
Abstract
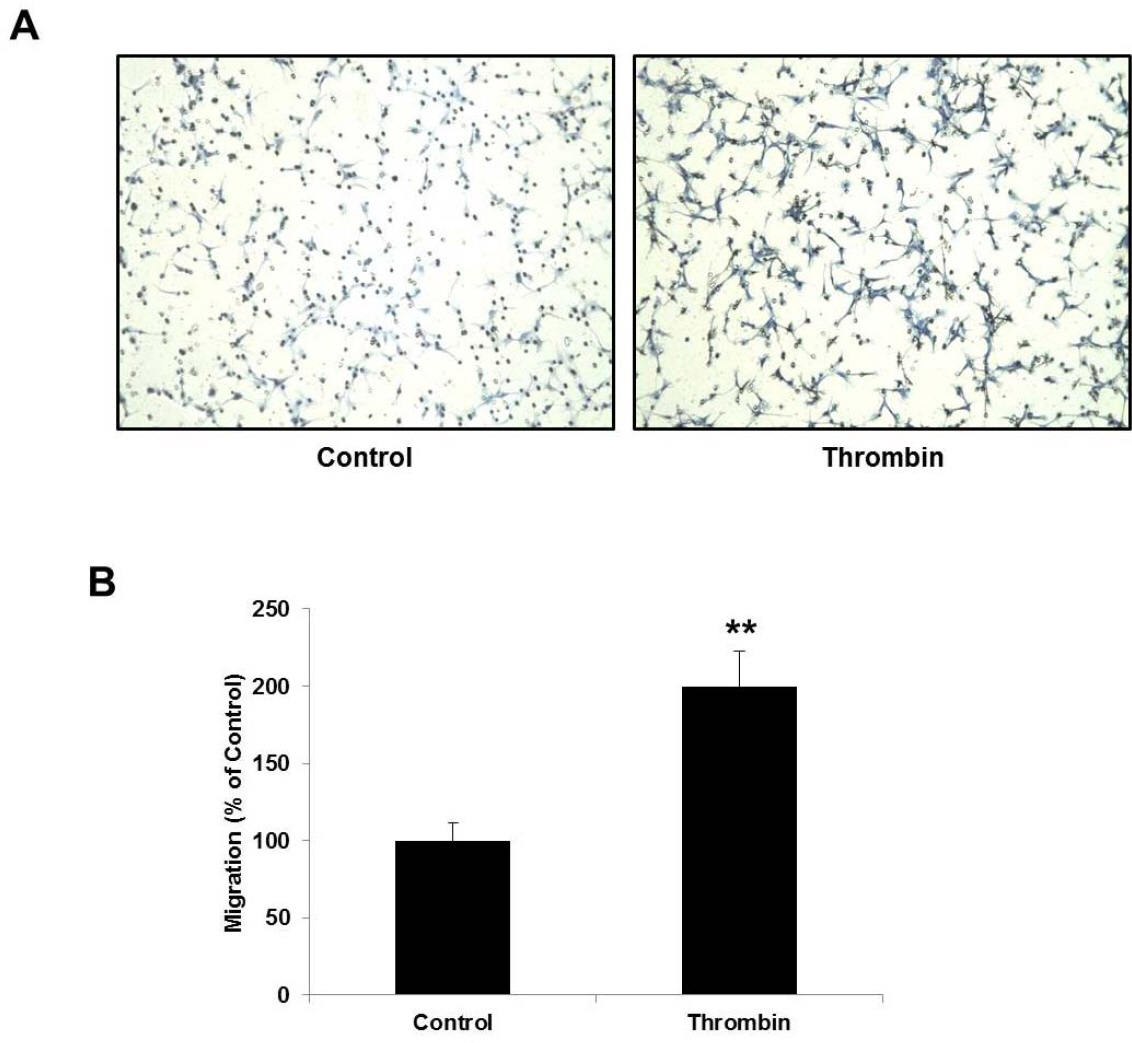
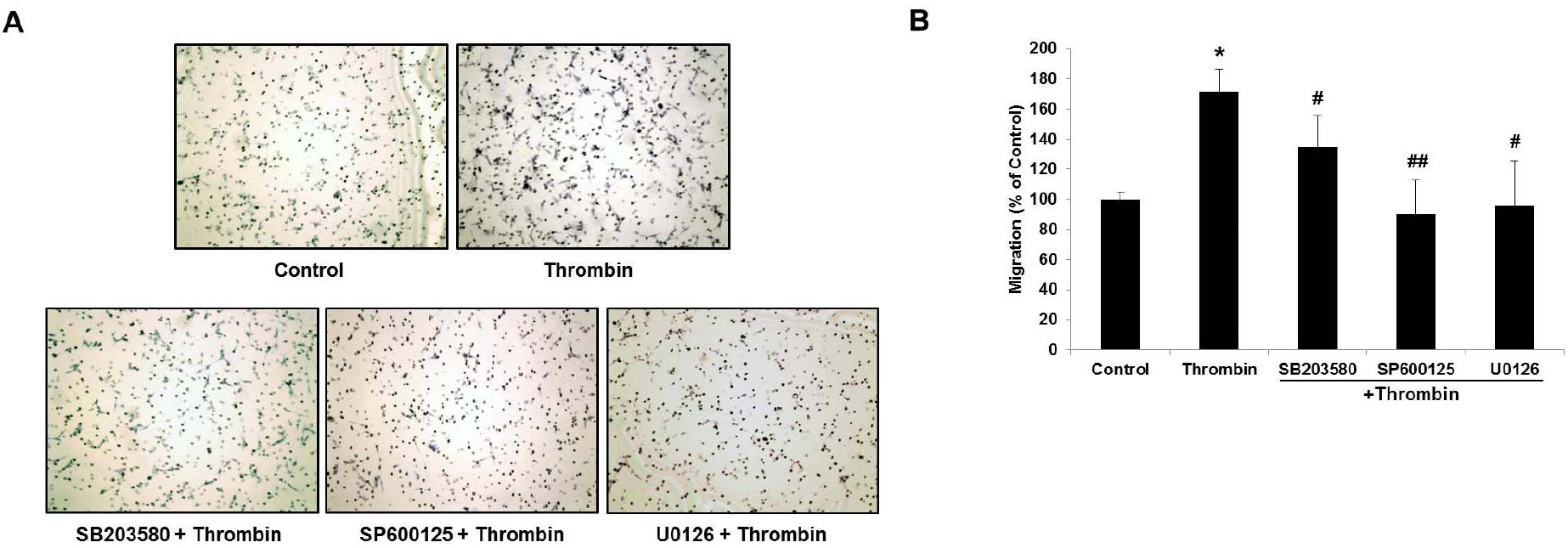
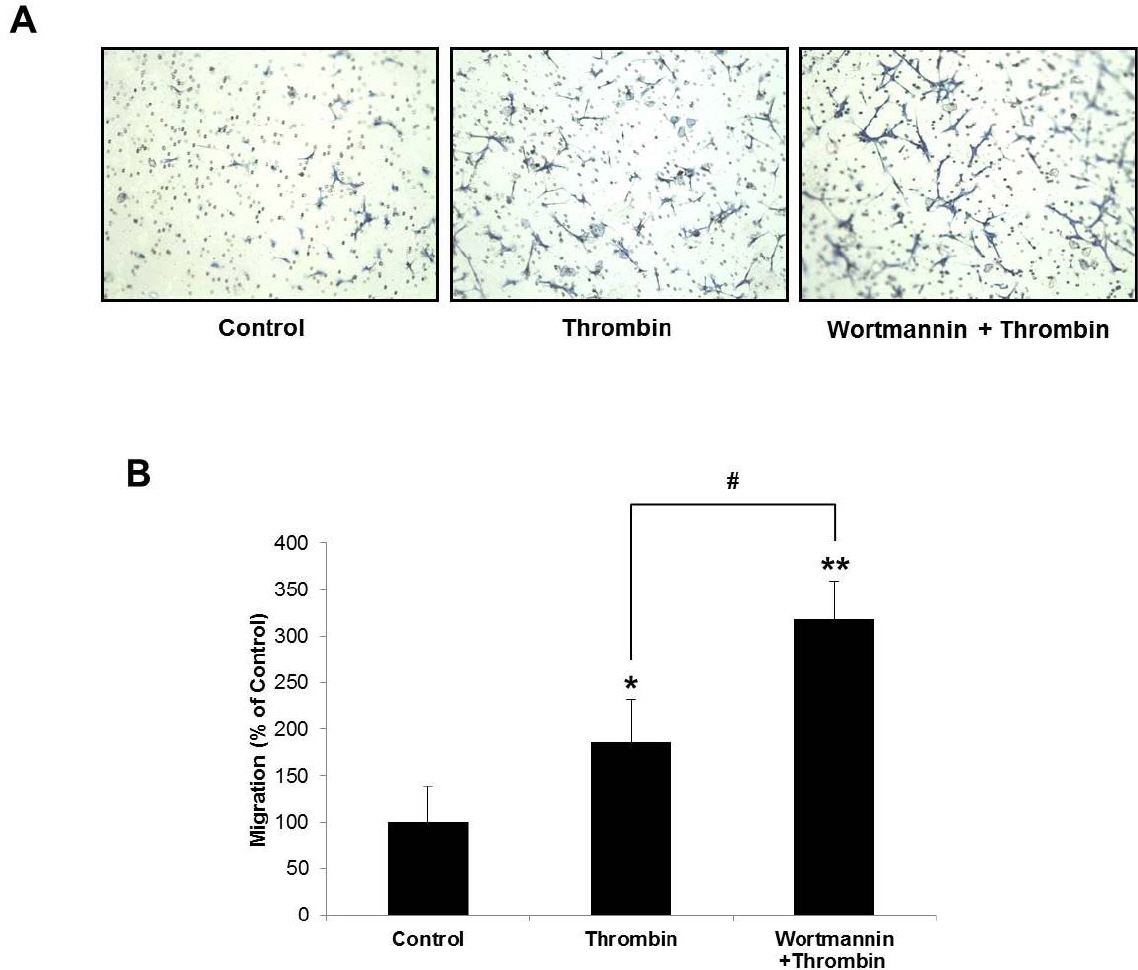
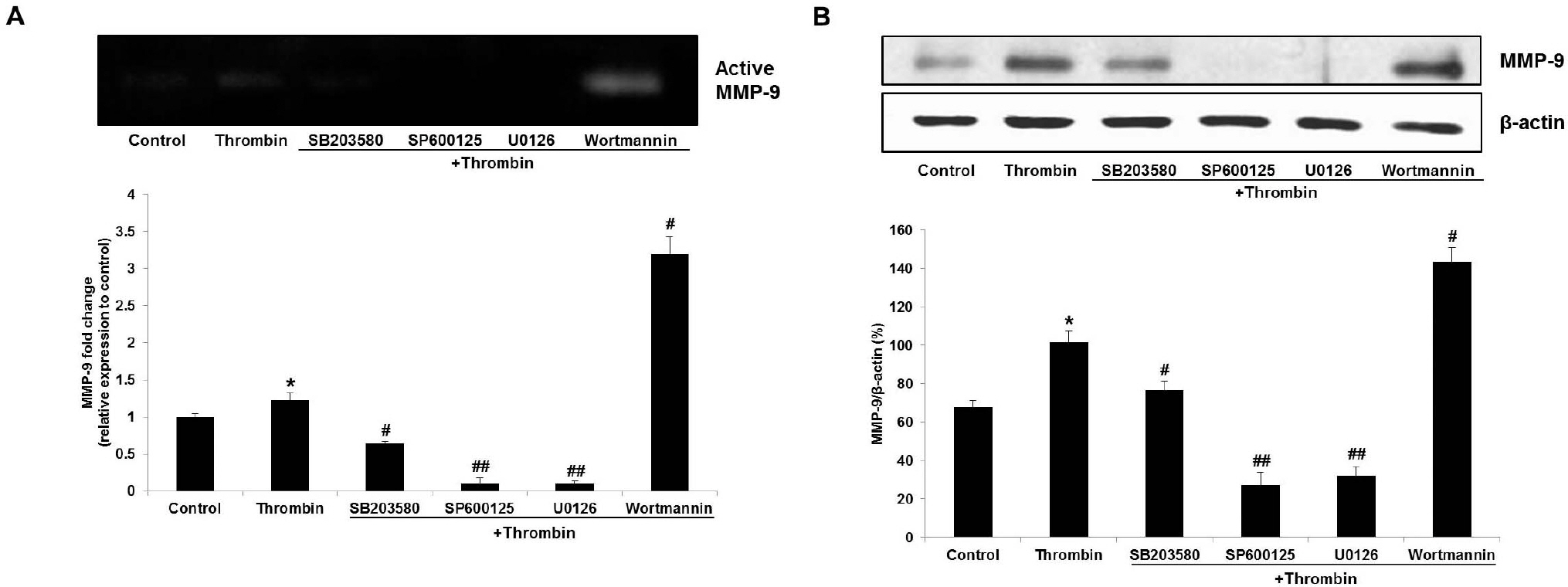
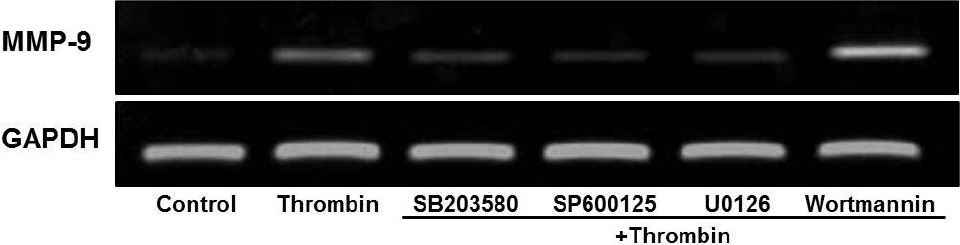
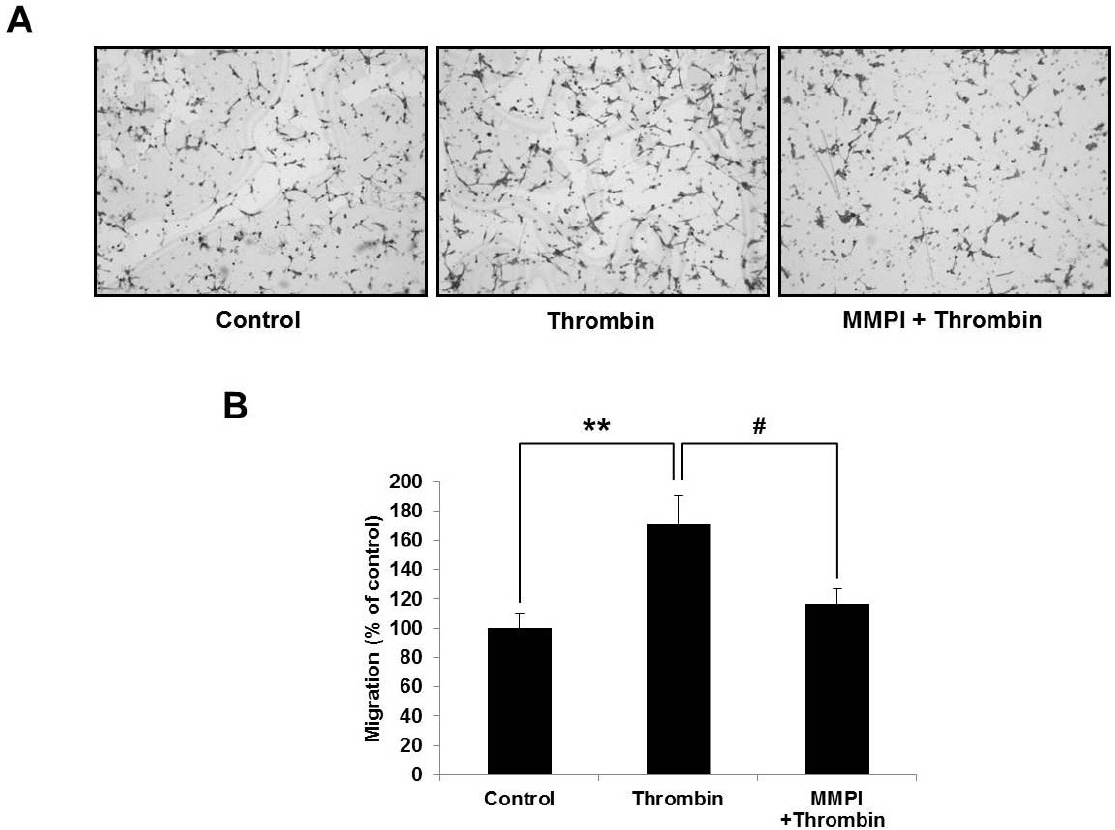
- Glioblastoma multiforme is one of the most common and aggressive tumors in central nervous system. It often possesses characteristic necrotic lesions with hemorrhages, which increase the chances of exposure to thrombin. Thrombin has been known as a regulator of MMP-9 expression and cancer cell migration. However, the effects of thrombin on glioma cells have not been clearly understood. In the present study, influences of thrombin on glioma cell migration were examined using Boyden chamber migration assay and thrombin-induced changes in MMP-9 expression were measured using zymography, semi-quantitative RT-PCR, and Western blotting. Furthermore, underlying signaling pathways by which thrombin induces MMP-9 expression were examined. Thrombin-induced migration and MMP-9 expression were significantly potentiated in the presence of wortmannin, a PI3K inhibitor, whereas MAPK inhibitors suppressed thrombin-induced migration and MMP-9 expression in C6 glioma cells. The present data strongly demonstrate that MAPK and PI3K pathways evidently regulate thrombin-induced migration and MMP-9 expression of C6 glioma cells. Therefore, the control of these pathways might be a beneficial therapeutic strategy for treatment of invasive glioblastoma multiforme.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.
Article2. Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005; 23:2411–2422.
Article3. Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA. 2000; 97:6242–6244.
Article4. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010; 141:52–67.
Article5. Björklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005; 1755:37–69.
Article6. Kawakita K, Kawai N, Kuroda Y, Yasashita S, Nagao S. Expression of matrix metalloproteinase-9 in thrombin-induced brain edema formation in rats. J Stroke Cerebrovasc Dis. 2006; 15:88–95.7. Choi MS, Kim YE, Lee WJ, Choi JW, Park GH, Kim SD, Jeon SJ, Go HS, Shin SM, Kim WK, Shin CY, Ko KH. Activation of protease-activated receptor1 mediates induction of matrix metalloproteinase-9 by thrombin in rat primary astrocytes. Brain Res Bull. 2008; 76:368–375.
Article8. Radjabi AR, Sawada K, Jagadeeswaran S, Eichbichler A, Kenny HA, Montag A, Bruno K, Lengyel E. Thrombin induces tumor invasion through the induction and association of matrix metalloproteinase-9 and beta1-integrin on the cell surface. J Biol Chem. 2008; 283:2822–2834.9. Chiang HS, Yang RS, Huang TF. Thrombin enhances the adhesion and migration of human colon adenocarcinoma cells via increased beta 3-integrin expression on the tumour cell surface and their inhibition by the snake venom peptide, rhodostomin. Br J Cancer. 1996; 73:902–908.10. Kaufmann R, Junker U, Junker K, Nuske K, Ranke C, Zieger M, Scheele J. The serine proteinase thrombin promotes migration of human renal carcinoma cells by a PKA-dependent mechanism. Cancer Lett. 2002; 180:183–190.
Article11. Heider I, Schulze B, Oswald E, Henklein P, Scheele J, Kaufmann R. PAR1-type thrombin receptor stimulates migration and matrix adhesion of human colon carcinoma cells by a PKCepsilon-dependent mechanism. Oncol Res. 2004; 14:475–482.12. Grobben B, De Deyn PP, Slegers H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002; 310:257–270.
Article13. Bizios R, Lai L, Fenton JW 2nd, Malik AB. Thrombin-induced chemotaxis and aggregation of neutrophils. J Cell Physiol. 1986; 128:485–490.
Article14. Pankonin G, Teuscher E. Stimulation of endothelial cell migration by thrombin. Biomed Biochim Acta. 1991; 50:1073–1078.15. Reddy KB, Nabha SM, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003; 22:395–403.16. Jadeski LC, Chakraborty C, Lala PK. Nitric oxide-mediated promotion of mammary tumour cell migration requires sequential activation of nitric oxide synthase, guanylate cyclase and mitogen-activated protein kinase. Int J Cancer. 2003; 106:496–504.
Article17. Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am J Surg. 2004; 188:560–5.
Article18. Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006; 93:E44–52.19. Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007; 19:11–7.
Article20. Kanu OO, Hughes B, Di C, Lin N, Fu J, Bigner DD, Yan H, Adamson C. Glioblastoma Multiforme Oncogenomics and Signaling Pathways. Clin Med Oncol. 2009; 3:39–52.
Article21. Chen HT, Tsou HK, Tsai CH, Kuo CC, Chiang YK, Chang CH, Fong YC, Tang CH. Thrombin enhanced migration and MMPs expression of human chondrosarcoma cells involves PAR receptor signaling pathway. J Cell Physiol. 2010; 223:737–745.
Article22. Wang Z, Castresana MR, Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004; 36:49–56.
Article23. Wang L, Luo J, He S. Induction of MMP-9 release from human dermal fibroblasts by thrombin: involvement of JAK/STAT3 signaling pathway in MMP-9 release. BMC Cell Biol. 2007; 8:14.
Article24. Watanabe H. Extracellular matrix–regulation of cancer invasion and metastasis. Gan To Kagaku Ryoho. 2010; 37:2058–2061.25. Estève PO, Robledo O, Potworowski EF, St-Pierre Y. Induced expression of MMP-9 in C6 glioma cells is inhibited by PDGF via a PI 3-kinase-dependent pathway. Biochem Biophys Res Commun. 2002; 296:864–869.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of yacon (Samallanthus sonchifolius) ethanol extract on cell proliferation and migration of C6 glioma cells stimulated with fetal bovine serum
- Compound K attenuates stromal cell-derived growth factor 1 (SDF-1)-induced migration of C6 glioma cells
- Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells
- Induction of Inducible Nitric Oxide Synthase Expression by Manganese in C6 Glioma Cells
- Dexamethasone Inhibits Interleukin-1beta-Induced Matrix Metalloproteinase-9 Expression in Cochlear Cells