Nutr Res Pract.
2015 Jun;9(3):256-261. 10.4162/nrp.2015.9.3.256.
The effect of yacon (Samallanthus sonchifolius) ethanol extract on cell proliferation and migration of C6 glioma cells stimulated with fetal bovine serum
- Affiliations
-
- 1Department of Medical Science, School of Medicine, Konkuk University, Seoul 143-701, Korea.
- 2Department of Anatomy, college of Korean Medicine, Dongguk University Gyeongju Campus 123, Dongdae-ro, Gyeongju-si, Gyengbuk, 780-714, Korea. pisdg@dongguk.ac.kr
- KMID: 2313838
- DOI: http://doi.org/10.4162/nrp.2015.9.3.256
Abstract
- BACKGROUND/OBJECTIVES
Yacon (Samallanthus sonchifolius), a common edible plant grown throughout the world, is well known for its antidiabetic properties. It is also known to have several other pharmacological properties including anti-inflammatory, anti-oxidant, anti-allergic, and anti-cancer effects. To date, the effect of yacon on gliomas has not been studied. In this study, we investigated the effects of yacon on the migration and proliferation of C6 glioma cells stimulated by fetal bovine serum (FBS).
MATERIALS/METHODS
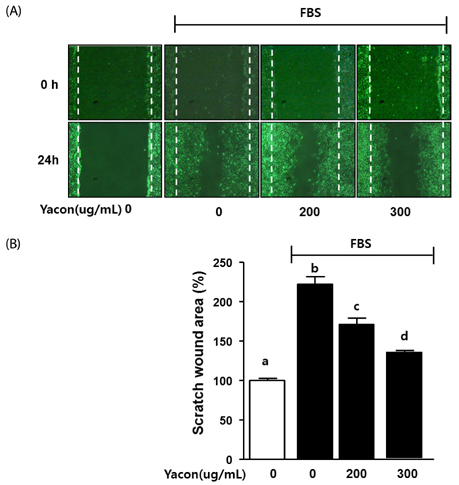
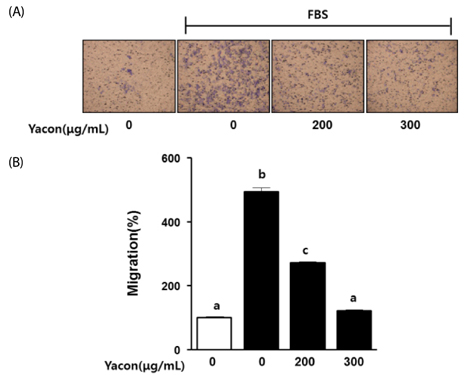
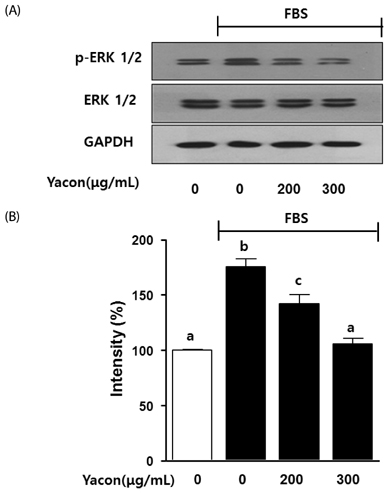
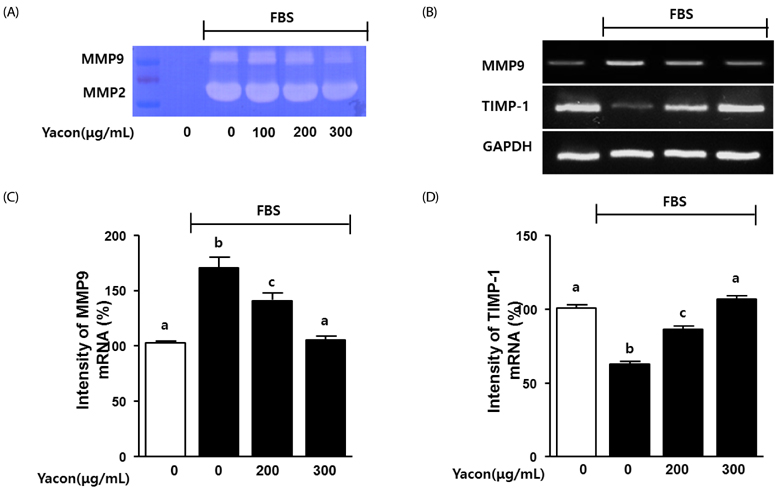
Cell growth and proliferation were determined by evaluating cell viability using an EZ-Cytox Cell Viability Assay Kit. FBS-induced migration of C6 glioma cells was evaluated by performing the scratch wound healing assay and the Boyden chamber assay. We also used western blot analysis to determine the expression levels of extracellular signal-regulated kinase 1/2 (ERK1/2), a major regulator of migration and proliferation of glioma cells. Matrix metallopeptidase (MMP) 9 and TIMP-1 levels were measured by performing reverse transcription PCR.
RESULTS
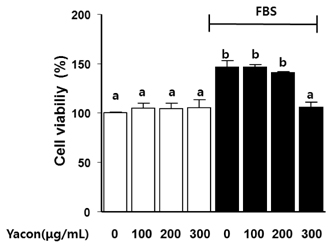
Yacon (300 microg/mL) reduced both the FBS-induced proliferation of C6 glioma cells and the dose-dependent migration of the FBS-stimulated C6 cells. FBS-stimulated C6 glioma cells treated with yacon (200 and 300 microg/mL) showed reduced phosphorylation of ERK1/2 and inhibition of MMP 9 expression compared to those shown by the untreated FBS-stimulated C6 cells. In contrast, yacon (200 and 300 microg/mL) induced TIMP-1 expression.
CONCLUSIONS
On the basis of these results, we suggest that yacon may exert an anti-cancer effect on FBS-stimulated C6 glioma cells by inhibiting their proliferation and migration. The most likely mechanism for this is down-regulation of ERK1/2 and MMP9 and up-regulation of TIMP-1 expression levels.
Keyword
MeSH Terms
-
Blotting, Western
Cell Proliferation*
Cell Survival
Down-Regulation
Ethanol*
Glioma*
Phosphorylation
Phosphotransferases
Plants, Edible
Polymerase Chain Reaction
Reverse Transcription
Tissue Inhibitor of Metalloproteinase-1
Up-Regulation
Wound Healing
Ethanol
Phosphotransferases
Tissue Inhibitor of Metalloproteinase-1
Figure
Cited by 1 articles
-
Compound K attenuates stromal cell-derived growth factor 1 (SDF-1)-induced migration of C6 glioma cells
Hyuck Kim, Hyo Sun Roh, Jai Eun Kim, Sun Dong Park, Won Hwan Park, Jin-Young Moon
Nutr Res Pract. 2016;10(3):259-264. doi: 10.4162/nrp.2016.10.3.259.
Reference
-
1. Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, Cloughesy TF. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013; 13:347.
Article2. Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977-2000. Cancer. 2004; 101:2293–2299.
Article3. Marucci G. The effect of WHO reclassification of necrotic anaplastic oligoastrocytomas on incidence and survival in glioblastoma. J Neurooncol. 2011; 104:621–622.
Article4. Coniglio SJ, Segall JE. Review: molecular mechanism of microglia stimulated glioblastoma invasion. Matrix Biol. 2013; 32:372–380.
Article5. Roy LO, Poirier MB, Fortin D. Transforming growth factor-beta and its implication in the malignancy of gliomas. Target Oncol. Forthcomng 2014.
Article6. Strnisková M, Barancík M, Ravingerová T. Mitogen-activated protein kinases and their role in regulation of cellular processes. Gen Physiol Biophys. 2002; 21:231–255.7. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001; 410:37–40.
Article8. Park SL, Kim WJ, Moon SK. p21WAF1 mediates the IL-15-induced migration and invasion of human bladder cancer 5637 cells via the ERK1/2/NF-kappaB/MMP-9 pathway. Int Immunopharmacol. 2014; 22:59–65.
Article9. Khasigov PZ, Podobed OV, Gracheva TS, Salbiev KD, Grachev SV, Berezov TT. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochemistry (Mosc). 2003; 68:711–717.10. Fang W, Li H, Kong L, Niu G, Gao Q, Zhou K, Zheng J, Wu B. Role of matrix metalloproteinases (MMPs) in tumor invasion and metastasis: serial studies on MMPs and TIMPs. Beijing Da Xue Xue Bao. 2003; 35:441–443.11. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25:9–34.
Article12. Delgado GT, Tamashiro WM, Maróstica MR Junior, Pastore GM. Yacon (Smallanthus sonchifolius): a functional food. Plant Foods Hum Nutr. 2013; 68:222–228.
Article13. Genta S, Cabrera W, Habib N, Pons J, Carillo IM, Grau A, Sánchez S. Yacon syrup: beneficial effects on obesity and insulin resistance in humans. Clin Nutr. 2009; 28:182–187.
Article14. de Almeida Paula HA, Abranches MV, de Luces Fortes Ferreira CL. Yacon (Smallanthus sonchifolius): a food with multiple functions. Crit Rev Food Sci Nutr. 2015; 55:32–40.
Article15. Piette C, Deprez M, Roger T, Noël A, Foidart JM, Munaut C. The dexamethasone-induced inhibition of proliferation, migration, and invasion in glioma cell lines is antagonized by macrophage migration inhibitory factor (MIF) and can be enhanced by specific MIF inhibitors. J Biol Chem. 2009; 284:32483–32492.
Article16. Kobayashi T, Hattori S, Shinkai H. Matrix metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol. 2003; 83:105–107.
Article17. Delgado GT, Thomé R, Gabriel DL, Tamashiro WM, Pastore GM. Yacon (Smallanthus sonchifolius)-derived fructooligosaccharides improves the immune parameters in the mouse. Nutr Res. 2012; 32:884–892.
Article18. Lin F, Hasegawa M, Kodama O. Purification and identification of antimicrobial sesquiterpene lactones from yacon (Smallanthus sonchifolius) leaves. Biosci Biotechnol Biochem. 2003; 67:2154–2159.
Article19. Martín V, Herrera F, García-Santos G, Antolín I, Rodriguez-Blanco J, Rodriguez C. Signaling pathways involved in antioxidant control of glioma cell proliferation. Free Radic Biol Med. 2007; 42:1715–1722.
Article20. Campos D, Betalleluz-Pallardel I, Chirinos R, Aguilar-Galvez A, Noratto G, Pedreschi R. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012; 135:1592–1599.
Article21. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003; 3:362–374.
Article22. Nagano M, Hoshino D, Koshikawa N, Akizawa T, Seiki M. Turnover of focal adhesions and cancer cell migration. Int J Cell Biol. 2012; 2012:310616.
Article23. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011; 278:16–27.
Article24. Li C, Wang F, Liu L. Ultra-high performance liquid chromatography-mass spectrometry for analysis of newborn and fetal bovine serum components. Nan Fang Yi Ke Da Xue Xue Bao. 2014; 34:751–753.25. Wang M, Maier P, Wenz F, Giordano FA, Herskind C. Mitogenic signalling in the absence of epidermal growth factor receptor activation in a human glioblastoma cell line. J Neurooncol. 2013; 115:323–331.
Article26. Ito Y, Correll K, Schiel JA, Finigan JH, Prekeris R, Mason RJ. Lung fibroblasts accelerate wound closure in human alveolar epithelial cells through hepatocyte growth factor/c-Met signaling. Am J Physiol Lung Cell Mol Physiol. 2014; 307:L94–L105.
Article27. Lin MC, Huang MJ, Liu CH, Yang TL, Huang MC. GALNT2 enhances migration and invasion of oral squamous cell carcinoma by regulating EGFR glycosylation and activity. Oral Oncol. 2014; 50:478–484.
Article28. Dong XZ, Huang CL, Yu BY, Hu Y, Mu LH, Liu P. Effect of Tenuifoliside A isolated from Polygala tenuifolia on the ERK and PI3K pathways in C6 glioma cells. Phytomedicine. 2014; 21:1178–1188.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Spermatogenic Effect of Yacon Extract and Its Constituents and Their Inhibition Effect of Testosterone Metabolism
- Proliferative Effect of Serum on Bovine Retinal Pigment Epithelial Cell Culture
- Effects of exogenous ATP on calcium mobilization and cell proliferation in C6 glioma cell
- Effect of Tamoxifen in C6 Glioma Cells
- The Effect of Yacon (Smallanthus sonchifolius) Extract against Dibutyltin Dichloride-induced Pancreatitis