Korean J Hematol.
2011 Dec;46(4):265-273. 10.5045/kjh.2011.46.4.265.
Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells
- Affiliations
-
- 1Department of Applied Bioscience, College of Life Science, CHA University, Sungnam, Korea. hoe@cha.ac.kr
- KMID: 2251988
- DOI: http://doi.org/10.5045/kjh.2011.46.4.265
Abstract
- BACKGROUND
The underlying rationale of platelet rich plasma (PRP) therapy is that an injection of concentrated PRP at the site of injury may promote tissue repair via cytokine release from platelets. The molecular mechanisms of PRP therapy in the skin wound healing process are not well understood at present, and would benefit from clarification.
METHODS
PRP was stimulated with angonists for 5 min, and cytokine profile analysis was performed. To investigate the wound healing activity of PRP, cell proliferation and migration analyses were performed in skin cells. The effects of PRP were analyzed on the expression and activity of matrix metalloproteinase (MMP)-1, -2, -9, and the activation of transcription factors.
RESULTS
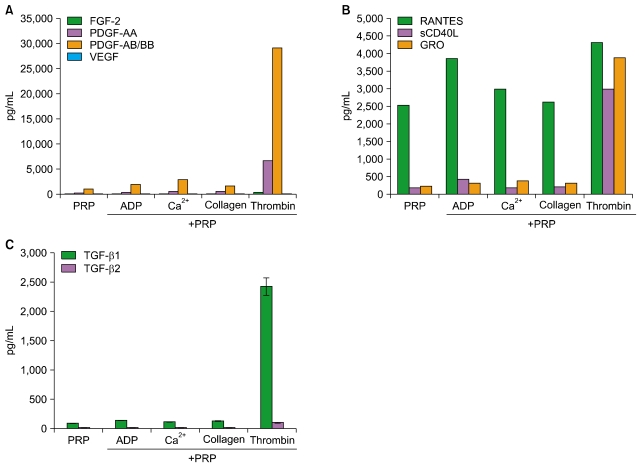
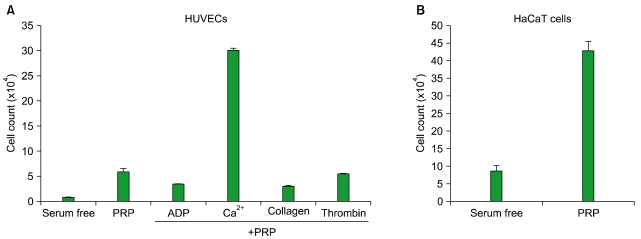
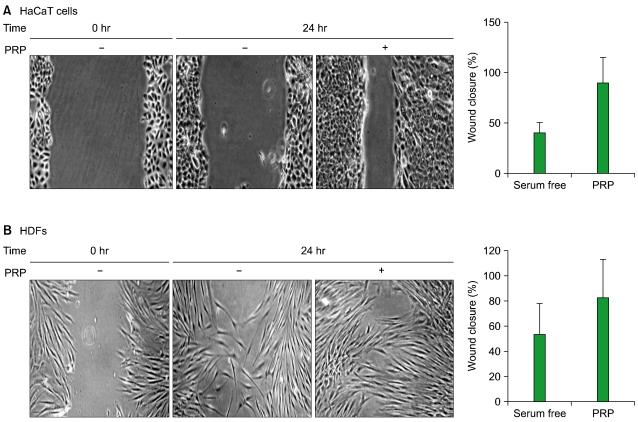
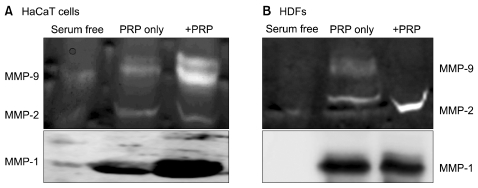
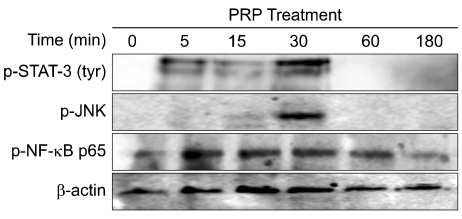
Thrombin was found to be a strong stimulator of PRP activation to release growth factors and chemokines. PRP induced cell proliferation and migration in HUVECs, HaCaT cells, and HDFs, as well as MMP-1and MMP-9 expression in HaCaT cells, but PRP did not have a significant effect on the expression or activity of MMPs in HDFs. The transcription factors, including signal transducer and activator of transcription-3 (STAT-3) were found to be phosphorylated following PRP treatment in HaCaT cells.
CONCLUSION
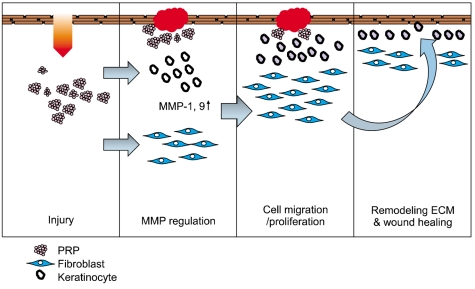
In this study, we have identified the cytokine profile of activated PRP after agonist stimulation. We have shown that PRP plays an active role in promoting the proliferation and migration of skin cells via the regulation of MMPs, and this may be applicable to the future development of PRP therapeutics to enhance skin wound healing.
Keyword
MeSH Terms
-
Blood Platelets
Cell Movement
Cell Proliferation
Chemokines
Intercellular Signaling Peptides and Proteins
Matrix Metalloproteinase 1
Matrix Metalloproteinases
Platelet-Rich Plasma
Skin
Thrombin
Transcription Factors
Transducers
Up-Regulation
Wound Healing
Chemokines
Intercellular Signaling Peptides and Proteins
Matrix Metalloproteinase 1
Matrix Metalloproteinases
Thrombin
Transcription Factors
Figure
Cited by 2 articles
-
Effects of granulocyte-colony stimulating factor and the expression of its receptor on various malignant cells
Hee Won Moon, Tae Young Kim, Bo Ra Oh, Sang Mee Hwang, Jiseok Kwon, Ja-Lok Ku, Dong Soon Lee
Korean J Hematol. 2012;47(3):219-224. doi: 10.5045/kjh.2012.47.3.219.Recombinant Human Granulocyte Colony-Stimulating Factor Promotes Preinvasive and Invasive Estrogen Receptor-Positive Tumor Development in MMTV-erbB2 Mice
Chun Ling Zhao, Guang Ping Zhang, Zheng Zheng Xiao, Zhi Kun Ma, Cai Peng Lei, Shi Yuan Song, Ying Ying Feng, Ya Chao Zhao, Xiao Shan Feng
J Breast Cancer. 2015;18(2):126-133. doi: 10.4048/jbc.2015.18.2.126.
Reference
-
1. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005; 111:3481–3488. PMID: 15983262.
Article2. Brass LF. Thrombin and platelet activation. Chest. 2003; 124(3 Suppl):18S–25S. PMID: 12970120.
Article3. Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006; 99:1293–1304. PMID: 17158345.
Article4. Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010; 74:597–607. PMID: 20197627.
Article5. Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009; 23:177–189. PMID: 19450911.6. Badimon L, Vilahur G, Padró T. Lipoproteins, platelets and atherothrombosis. Rev Esp Cardiol. 2009; 62:1161–1178. PMID: 19793522.7. Cattaneo M, Gachet C. ADP receptors and clinical bleeding disorders. Arterioscler Thromb Vasc Biol. 1999; 19:2281–2285. PMID: 10521355.
Article8. Jin J, Quinton TM, Zhang J, Rittenhouse SE, Kunapuli SP. Adenosine diphosphate (ADP)-induced thromboxane A(2) generation in human platelets requires coordinated signaling through integrin alpha(IIb)beta(3) and ADP receptors. Blood. 2002; 99:193–198. PMID: 11756171.9. Toriseva M, Kähäri VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009; 66:203–224. PMID: 18810321.
Article10. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993; 362:801–809. PMID: 8479518.
Article11. Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995; 25(Suppl 2):S9–S12. PMID: 8699871.
Article12. Ganz P, Creager MA, Fang JC, et al. Pathogenic mechanisms of atherosclerosis: effect of lipid lowering on the biology of atherosclerosis. Am J Med. 1996; 101:4A10S–4A16S.13. Lee E, Vaughan DE, Parikh SH, et al. Regulation of matrix metalloproteinases and plasminogen activator inhibitor-1 synthesis by plasminogen in cultured human vascular smooth muscle cells. Circ Res. 1996; 78:44–49. PMID: 8603504.
Article14. Wang X, Qiu Y, Triffitt J, Carr A, Xia Z, Sabokbar A. Proliferation and differentiation of human tenocytes in response to platelet rich plasma: An in vitro and In vivo study. J Orthop Res. 2011; [Epub ahead of print].
Article15. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008; 36:1171–1178. PMID: 18326832.16. Caceres M, Martinez C, Martinez J, Smith PC. Effects of platelet-rich and -poor plasma on the reparative response of gingival fibroblasts. Clin Oral Implants Res. 2011; [Epub ahead of print].17. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010; 303:144–149. PMID: 20068208.18. Ranzato E, Patrone M, Pedrazzi M, Burlando B. Hmgb1 promotes wound healing of 3T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem Biophys. 2010; 57:9–17. PMID: 20361273.
Article19. Lova P, Campus F, Lombardi R, et al. Contribution of protease-activated receptors 1 and 4 and glycoprotein Ib-IX-V in the G(i)-independent activation of platelet Rap1B by thrombin. J Biol Chem. 2004; 279:25299–25306. PMID: 15078882.
Article20. Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003; 10:335–350. PMID: 12851650.
Article21. Henn V, Steinbach S, Büchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001; 98:1047–1054. PMID: 11493450.
Article22. Mellembakken JR, Solum NO, Ueland T, Videm V, Aukrust P. Increased concentrations of soluble CD40 ligand, RANTES and GRO-alpha in preeclampsia-possible role of platelet activation. Thromb Haemost. 2001; 86:1272–1276. PMID: 11816717.23. Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005; 304:274–286. PMID: 15707592.
Article24. Lawson JH. The clinical use and immunologic impact of thrombin in surgery. Semin Thromb Hemost. 2006; 32(Suppl 1):98–110. PMID: 16673271.
Article25. Schneider L, Cammer M, Lehman J, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010; 25:279–292. PMID: 20110689.
Article26. Lederle W, Stark HJ, Skobe M, Fusenig NE, Mueller MM. Platelet-derived growth factor-BB controls epithelial tumor phenotype by differential growth factor regulation in stromal cells. Am J Pathol. 2006; 169:1767–1783. PMID: 17071599.
Article27. Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007; 102:593–608. PMID: 17729308.28. Joo CK, Seomun Y. Matrix metalloproteinase (MMP) and TGF beta 1-stimulated cell migration in skin and cornea wound healing. Cell Adh Migr. 2008; 2:252–253. PMID: 19262153.29. Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002; 2:657–672. PMID: 12209155.
Article30. Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007; 18:639–648. PMID: 17590158.
Article31. Su CY, Kuo YP, Nieh HL, Tseng YH, Burnouf T. Quantitative assessment of the kinetics of growth factors release from platelet gel. Transfusion. 2008; 48:2414–2420. PMID: 18694465.
Article32. Sanchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009; 39:345–354. PMID: 19402740.33. Harrison S, Vavken P, Kevy S, Jacobson M, Zurakowski D, Murray MM. Platelet activation by collagen provides sustained release of anabolic cytokines. Am J Sports Med. 2011; 39:729–734. PMID: 21398575.
Article34. Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005; 25:1512–1518. PMID: 15890969.35. VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003; 59:277–287. PMID: 12909311.
Article36. Choi WS, Jeon OH, Kim DS. CD40 ligand shedding is regulated by interaction between matrix metalloproteinase-2 and platelet integrin alpha(IIb)beta(3). J Thromb Haemost. 2010; 8:1364–1371. PMID: 20230421.37. Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Müller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998; 80:1008–1014. PMID: 9869175.
Article38. Simsek S, Folman C, van der Schoot CE, von dem Borne AE. The Arg633His substitution responsible for the private platelet antigen Gro(a) unravelled by SSCP analysis and direct sequencing. Br J Haematol. 1997; 97:330–335. PMID: 9163597.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Expression of Type I Collagen, MMP-1, and MMP-2 in Platelet-rich Plasma-treated Human Skin Fibroblasts
- Therapeutic efficacy of TriCell CD34+ cell-containing, platelet-rich plasma in alopecia patients
- The use of platelet-rich plasma in management of musculoskeletal pain: a narrative review
- The Efficacy and Safety of Platelet-Rich Plasma and Adipose-Derived Stem Cells: An Update
- The Effect of Platelet-rich Plasma on Wounds of OLETF Rats Using Expression of Matrix Metalloproteinase-2 and -9 mRNA