5-Hydroxytryptamine Generates Tonic Inward Currents on Pacemaker Activity of Interstitial Cells of Cajal from Mouse Small Intestine
- Affiliations
-
- 1Department of Physiology, College of Medicine, Chosun University, Gwangju 501-759, Korea. jyjun@chosun.ac.kr
- 2Department of Internal Medicine, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 3Department of Psychiatry, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- KMID: 1448915
- DOI: http://doi.org/10.4196/kjpp.2011.15.3.129
Abstract
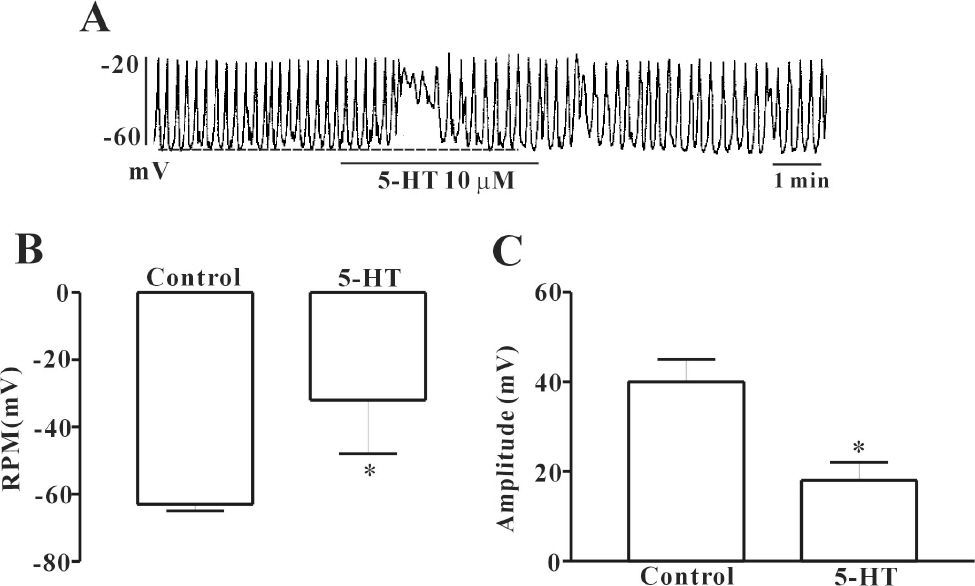
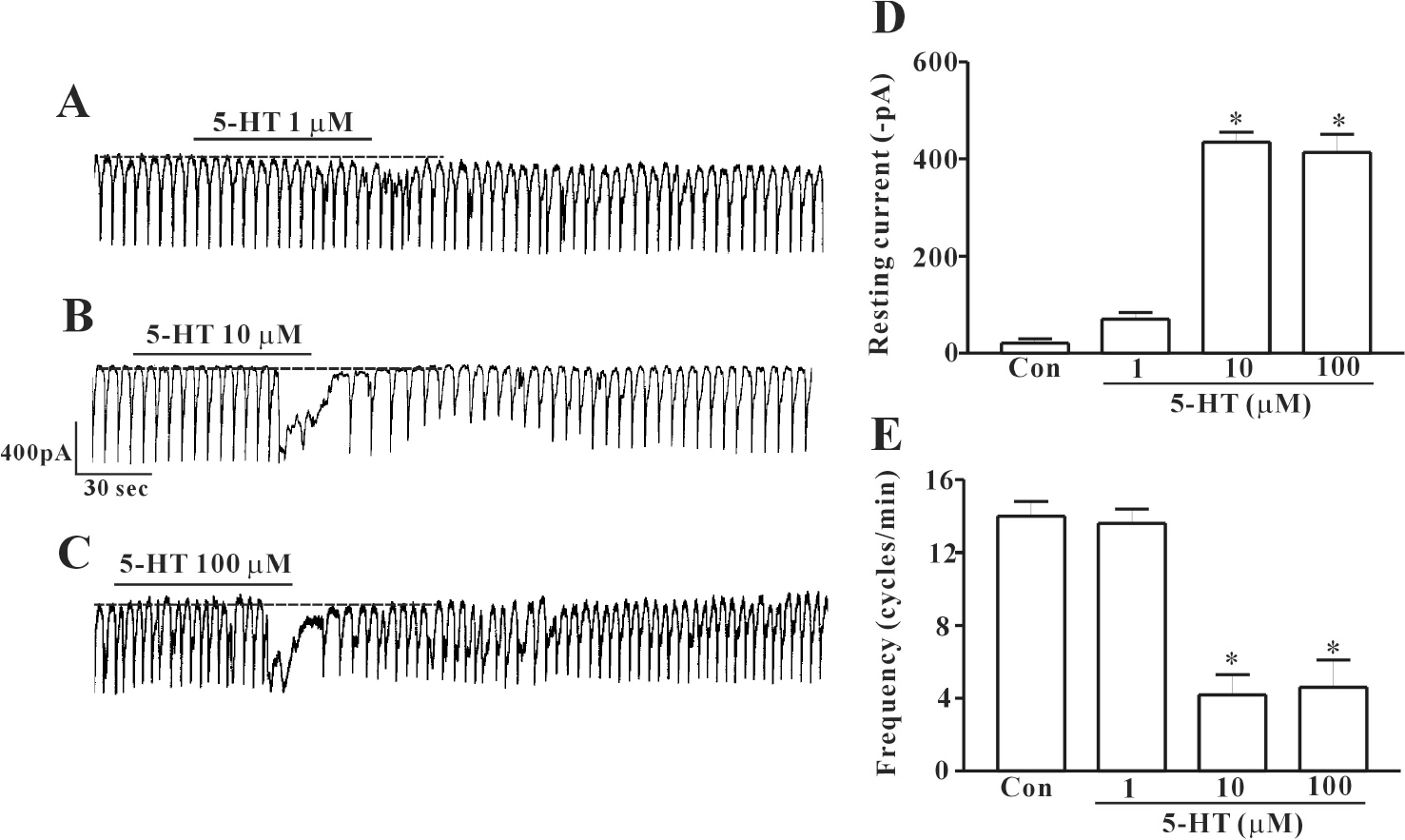
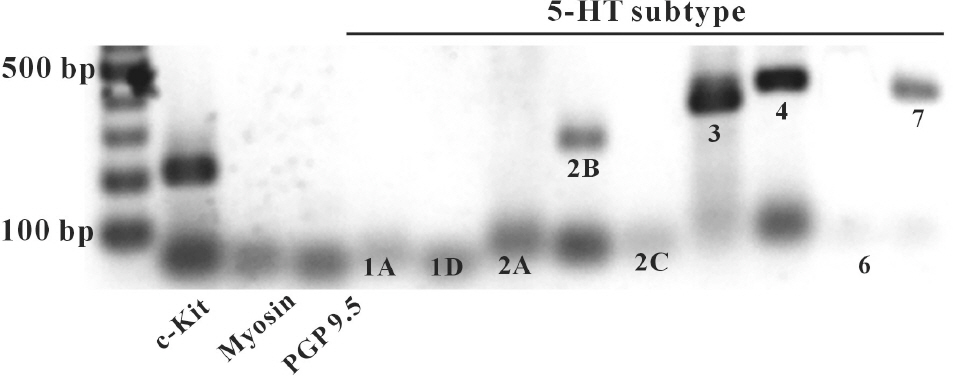
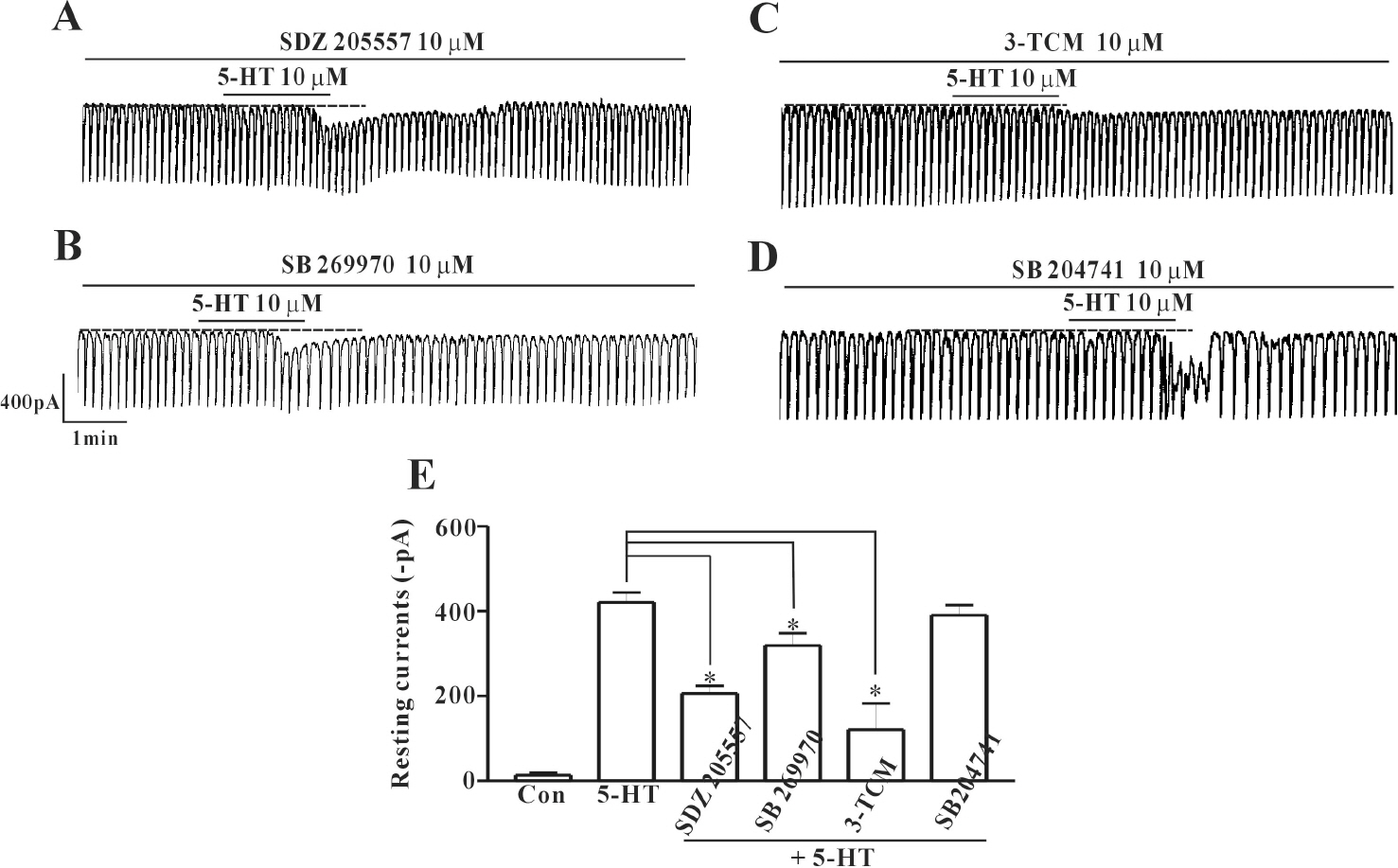
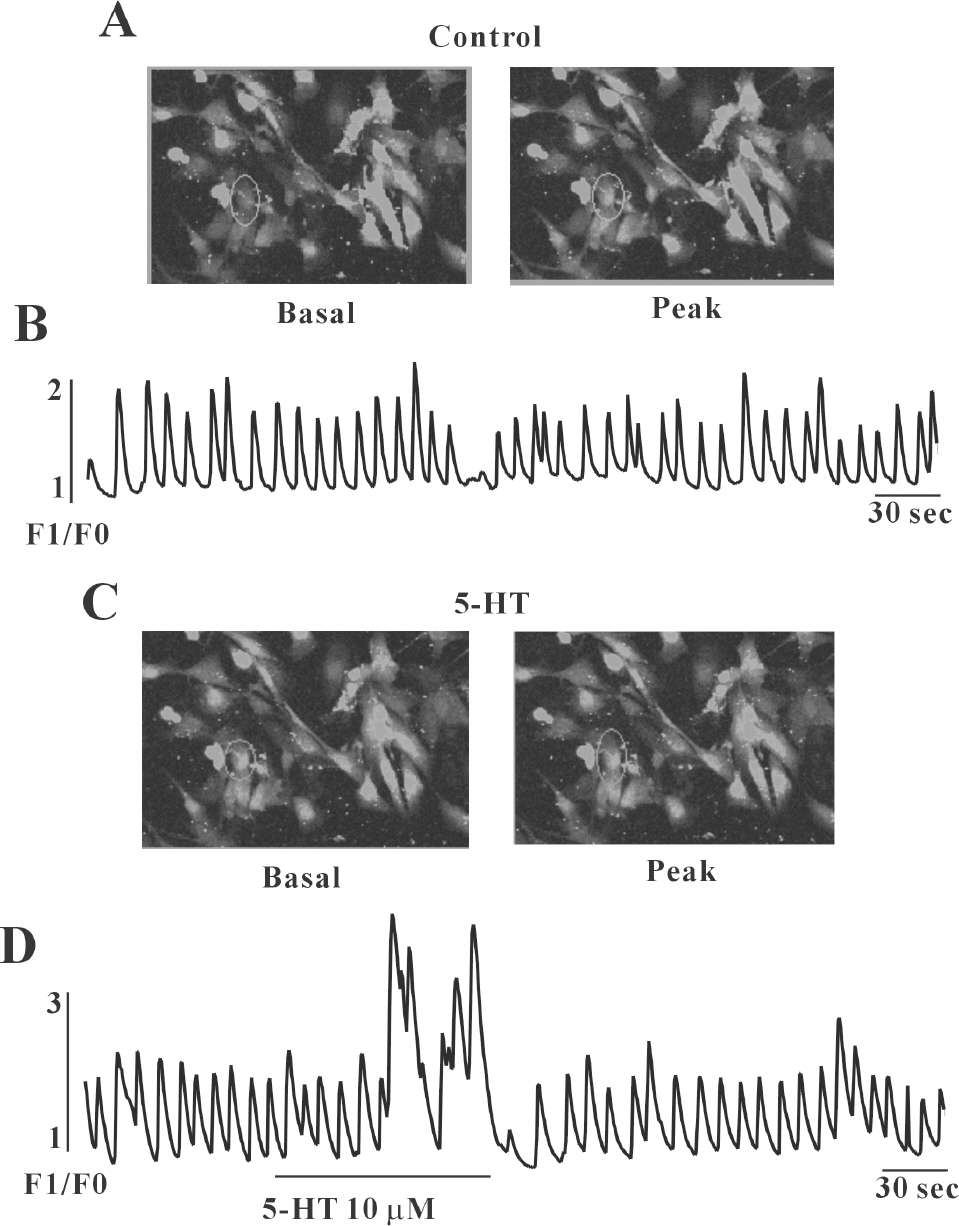
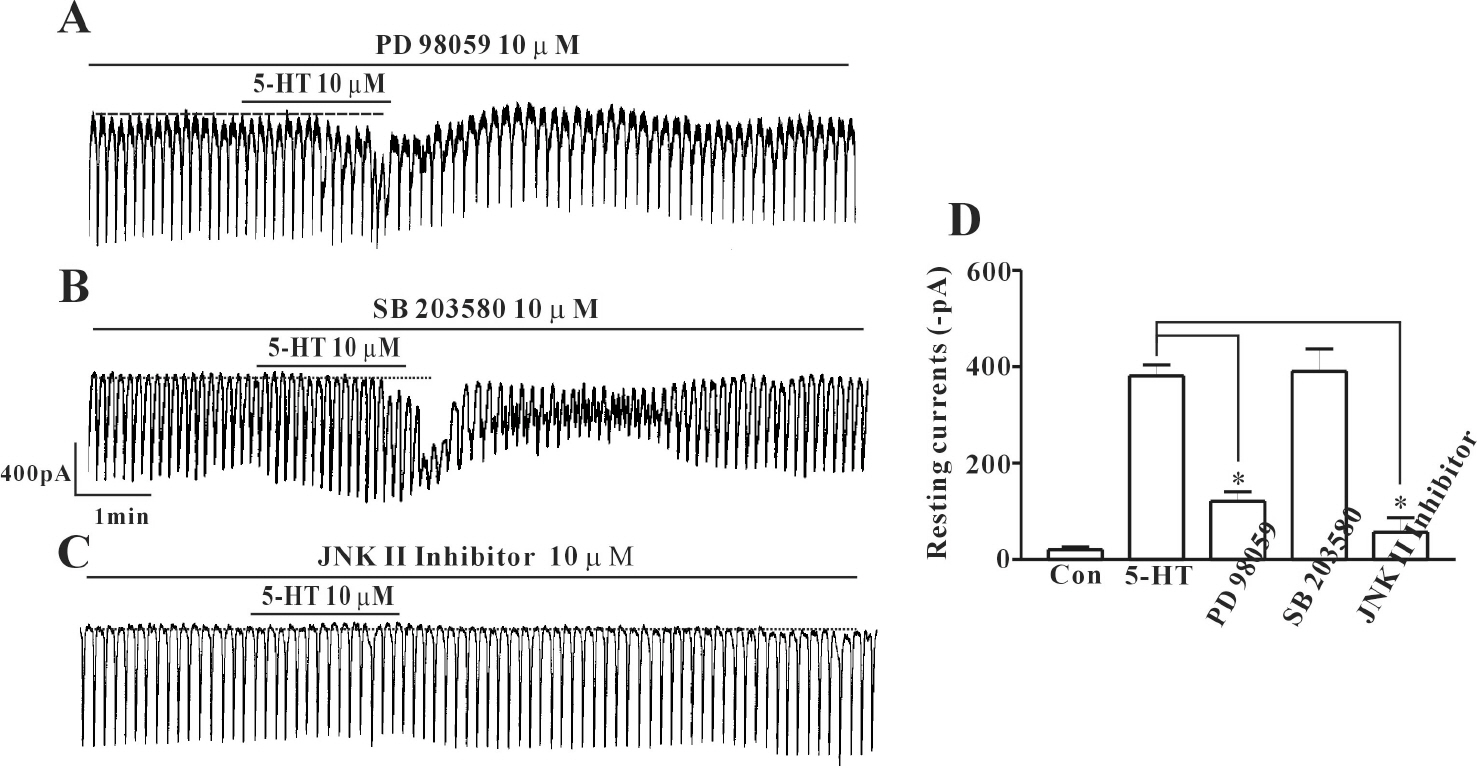
- In this study we determined whether or not 5-hydroxytryptamine (5-HT) has an effect on the pacemaker activities of interstitial cells of Cajal (ICC) from the mouse small intestine. The actions of 5-HT on pacemaker activities were investigated using a whole-cell patch-clamp technique, intracellular Ca2+ ([Ca2+]i) analysis, and RT-PCR in ICC. Exogenously-treated 5-HT showed tonic inward currents on pacemaker currents in ICC under the voltage-clamp mode in a dose-dependent manner. Based on RT-PCR results, we found the existence of 5-HT2B, 3, 4, and 7 receptors in ICC. However, SDZ 205557 (a 5-HT4 receptor antagonist), SB 269970 (a 5-HT7 receptor antagonist), 3-tropanylindole - 3 - carboxylate methiodide (3-TCM; a 5-HT3 antagonist) blocked the 5-HT-induced action on pacemaker activity, but not SB 204741 (a 5-HT2B receptor antagonist). Based on [Ca2+]i analysis, we found that 5-HT increased the intensity of [Ca2+]i. The treatment of PD 98059 or JNK II inhibitor blocked the 5-HT-induced action on pacemaker activity of ICC, but not SB 203580. In summary, these results suggest that 5-HT can modulate pacemaker activity through 5-HT3, 4, and 7 receptors via [Ca2+]i mobilization and regulation of mitogen-activated protein kinases.
Keyword
MeSH Terms
-
Animals
Flavonoids
Gastrointestinal Motility
Imidazoles
Interstitial Cells of Cajal
Intestine, Small
Mice
Mitogen-Activated Protein Kinases
para-Aminobenzoates
Patch-Clamp Techniques
Phenols
Pyridines
Receptor, Serotonin, 5-HT2B
Receptors, Serotonin
Receptors, Serotonin, 5-HT4
Serotonin
Sulfonamides
Flavonoids
Imidazoles
Mitogen-Activated Protein Kinases
Phenols
Pyridines
Receptor, Serotonin, 5-HT2B
Receptors, Serotonin
Receptors, Serotonin, 5-HT4
Serotonin
Sulfonamides
para-Aminobenzoates
Figure
Cited by 3 articles
-
Effects of Histamine on Cultured Interstitial Cells of Cajal in Murine Small Intestine
Byung Joo Kim, Young Kyu Kwon, Euiyong Kim, Insuk So
Korean J Physiol Pharmacol. 2013;17(2):149-156. doi: 10.4196/kjpp.2013.17.2.149.Spontaneous Electrical Activity of Cultured Interstitial Cells of Cajal from Mouse Urinary Bladder
Sun-Ouck Kim, Han-Seong Jeong, Sujeong Jang, Mei-Jin Wu, Jong Kyu Park, Han-Yi Jiao, Jae Yeoul Jun, Jong-Seong Park
Korean J Physiol Pharmacol. 2013;17(6):531-536. doi: 10.4196/kjpp.2013.17.6.531.Pituitary Adenylate Cyclase-activating Polypeptide Inhibits Pacemaker Activity of Colonic Interstitial Cells of Cajal
Mei Jin Wu, Keun Hong Kee, Jisun Na, Seok Won Kim, Youin Bae, Dong Hoon Shin, Seok Choi, Jae Yeoul Jun, Han-Seong Jeong, Jong-Seong Park
Korean J Physiol Pharmacol. 2015;19(5):435-440. doi: 10.4196/kjpp.2015.19.5.435.
Reference
-
References
1. Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995; 269:C1577–1585.
Article2. Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995; 373:347–349.3. Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999; 38:1083–1152.
Article4. Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002; 71:533–554.
Article5. Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001; 530:431–442.
Article6. Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J Neurosci. 2000; 20:294–305.7. Nebigil CG, Etienne N, Messaddeq N, Maroteaux L. Serotonin is a novel survival factor of cardiomyocytes: mitochondria as a target of 5-HT2B receptor signaling. FASEB J. 2003; 17:1373–1375.8. Baker DE. Rationale for using serotonergic agents to treat irritable bowel syndrome. Am J Health Syst Pharm. 2005; 62:700–711.
Article9. Hansen MB, Skadhauge E. Signal transduction pathways for serotonin as an intestinal secretagogue. Comp Biochem Physiol A Physiol. 1997; 118:283–290.
Article10. Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999; 288:93–97.11. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007; 132:397–414.
Article12. Read NW, Gwee KA. The importance of 5-hydroxytryptamine receptors in the gut. Pharmacol Ther. 1994; 62:159–173.
Article13. Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007; 133:897–906.14. Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol. 2008; 377:181–203.
Article15. Epperson A, Hatton WJ, Callaghan B, Doherty P, Walker RL, Sanders KM, Ward SM, Horowitz B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol Cell Physiol. 2000; 279:C529–539.
Article16. Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR Jr, Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002; 123:217–226.17. De Ponti F. Pharmacology of serotonin: what a clinician should know. Gut. 2004; 53:1520–1535.
Article18. Gershon MD. Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004; 20 Suppl. 7:3–14.
Article19. Buchheit KH, Buhl T. Stimulant effects of 5-hydroxytryptamine on guinea pig stomach preparations in vitro. Eur J Pharmacol. 1994; 262:91–97.20. Xue L, Camilleri M, Locke GR 3rd, Schuurkes JA, Meulemans A, Coulie BJ, Szurszewski JH, Farrugia G. Serotonergic modulation of murine fundic tone. Am J Physiol Gastrointest Liver Physiol. 2006; 291:G1180–1186.
Article21. Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000; 525:355–361.
Article22. Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000; 525:105–111.
Article23. Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SR, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004; 117:2813–2825.24. Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, So I, Kim KW, Jun JY. Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006; 18:187–198.25. So KY, Kim SH, Sohn HM, Choi SJ, Parajuli SP, Choi S, Yeum CH, Yoon PJ, Jun JY. Carbachol regulates pacemaker activities in cultured interstitial cells of Cajal from the mouse small intestine. Mol Cells. 2009; 27:525–531.
Article26. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001; 22:153–183.
Article27. Errico M, Crozier RA, Plummer MR, Cowen DS. 5-HT(7) receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience. 2001; 102:361–367.
Article28. Machida T, Ohta M, Onoguchi A, Iizuka K, Sakai M, Minami M, Hirafuji M. 5-Hydroxytryptamine induces cyclooxygenase-2 in rat vascular smooth muscle cells: mechanisms involving Src, PKC and MAPK activation. Eur J Pharmacol. 2011; 656:19–26.29. Park CG, Kim YD, Kim MY, Koh JW, Jun JY, Yeum CH, So I, Choi S. Effects of prostaglandin F2α on small intestinal interstitial cells of Cajal. World J Gastroenterol. 2011; 17:1143–1151.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Bradykinin Receptors and Effects of Bradykinin in the Interstitial Cells of Cajal from Mouse Small Intestine
- Effects of ATP on Pacemaker Activity of Interstitial Cells of Cajal from the Mouse Small Intestine
- Interplay of Hydrogen Sulfide and Nitric Oxide on the Pacemaker Activity of Interstitial Cells of Cajal from Mouse Small Intestine
- Inhibition of Pacemaker Activity of Interstitial Cells of Cajal by Hydrogen Peroxide via Activating ATP-sensitive K(+) Channels
- The Inhibitory Effects of Hydrogen Sulfide on Pacemaker Activity of Interstitial Cells of Cajal from Mouse Small Intestine