Anat Cell Biol.
2011 Dec;44(4):304-313. 10.5115/acb.2011.44.4.304.
The use of Stronger Neo-Minophagen C, a glycyrrhizin-containing preparation, in robust neuroprotection in the postischemic brain
- Affiliations
-
- 1Department of Anatomy, Center for Advanced Medical Education by BK21 Project, Inha University School of Medicine, Incheon, Korea. jklee@inha.ac.kr
- 2Department of Anatomy, Jeju National University School of Medicine, Jeju, Korea.
- KMID: 1447444
- DOI: http://doi.org/10.5115/acb.2011.44.4.304
Abstract
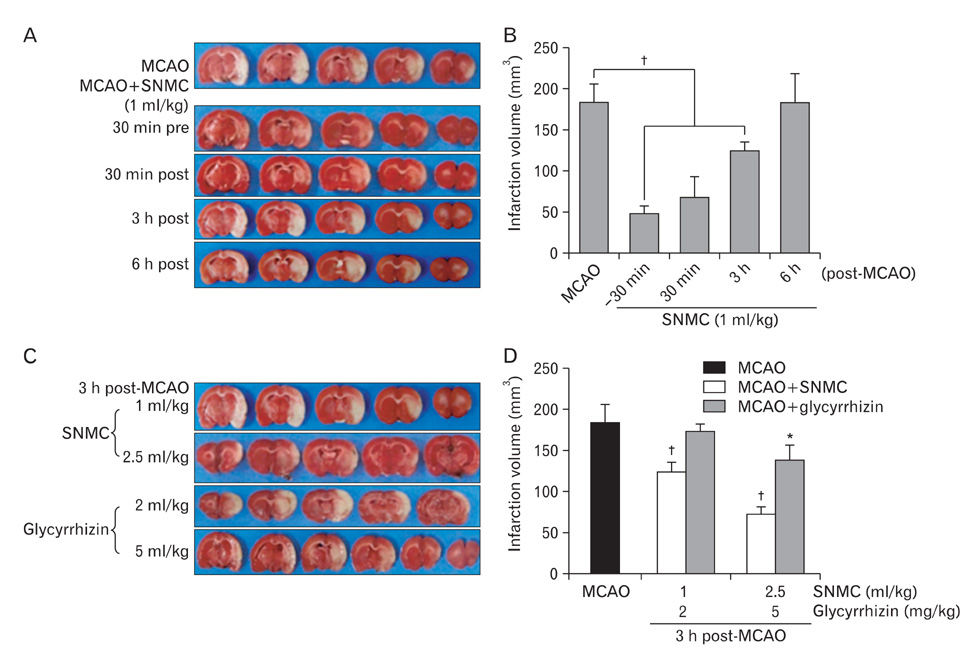
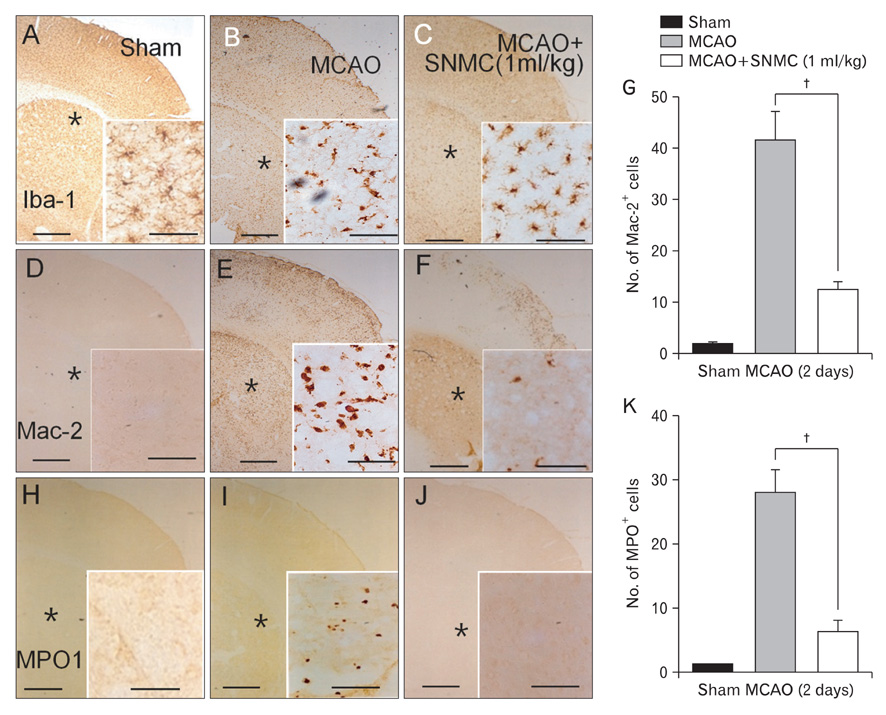
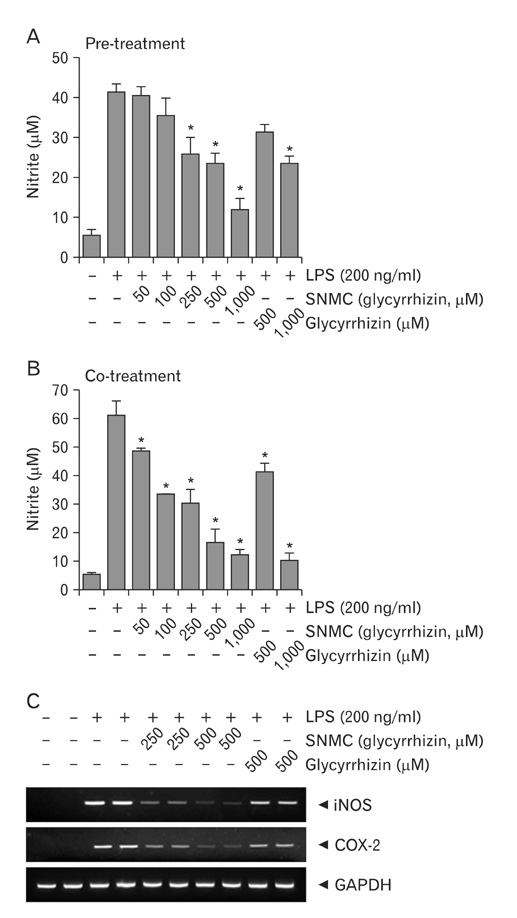
- Stronger Neo-Minophagen C (SNMC) is a glycyrrhizin-containing preparation that is approved in Japan for the treatment of chronic hepatic diseases and is marketed in Japan, China, Korea, Taiwan, and India. Glycyrrhizin, a triterpene present in the roots and rhizomes of licorice (Glycyrrhiza glabra) has been shown to have anti-inflammatory, anti-oxidative, and anti-viral effects. In the present study, we demonstrated the marked neuroprotective effects of SNMC in the postischemic rat brain after middle cerebral artery occlusion (MCAO). We used 1 ml/kg of SNMC, which is within the dose range used for the treatment of patients with chronic hepatic disease. The administration of SNMC intravenously at 30 minutes before or 30 minutes and 3 hours after MCAO (60 minutes) reduces mean infarct volumes to 27.0+/-4.2%, 37.1+/-12.4%, and 67.8+/-5.8% of that of untreated controls, respectively. This neuroprotective effect is accompanied by improvements in motor impairment and neurological deficits. The administration of SNMC is shown to suppress microglia activation and neutrophil infiltration in the postischemic brain. In addition, SNMC suppresses lipopolysaccharide-induced nitrite production and proinflammatory cytokine induction in a microglia cell line, BV2. This indicates that the neuroprotective effect of SNMC might be due, at least in part, to an anti-inflammatiory effect. Interestingly, SNMC shows significantly higher neuroprotective potency compared to an equivalent dose of pure glycyrrhizin, in terms of reducing infarct volume and improving neurological deficits. Together these results indicate that SNMC, a glycyrrhizin-containing preparation developed for chronic liver disease, has a marked neuroprotective function in the postischemic brain via its anti-inflammatory effects.
Keyword
MeSH Terms
-
Animals
Brain
Cell Line
China
Cysteine
Drug Combinations
Glycine
Glycyrrhetinic Acid
Glycyrrhiza
Glycyrrhizic Acid
Humans
India
Infarction, Middle Cerebral Artery
Japan
Korea
Liver Diseases
Microglia
Neuroprotective Agents
Neutrophil Infiltration
Rats
Rhizome
Taiwan
Cysteine
Drug Combinations
Glycine
Glycyrrhetinic Acid
Glycyrrhizic Acid
Neuroprotective Agents
Figure
Reference
-
1. Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999. 79:1431–1568.2. Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001. 21:99–109.3. Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982. 239:57–69.4. Michaelis M, Geiler J, Naczk P, Sithisarn P, Leutz A, Doerr HW, Cinatl J Jr. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS One. 2011. 6:e19705.5. Orlent H, Hansen BE, Willems M, Brouwer JT, Huber R, Kullak-Ublick GA, Gerken G, Zeuzem S, Nevens F, Tielemans WC, Zondervan PE, Lagging M, Westin J, Schalm SW. Biochemical and histological effects of 26 weeks of glycyrrhizin treatment in chronic hepatitis C: a randomized phase II trial. J Hepatol. 2006. 45:539–546.6. Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997. 79:1494–1500.7. Finney RS, Somers GF. The antiinflammatory activity of glycyrrhetinic acid and derivatives. J Pharm Pharmacol. 1958. 10:613–620.8. Iino S, Tango T, Matsushima T, Toda G, Miyake K, Hino K, Kumada H, Yasuda K, Kuroki T, Hirayama C, Suzuki H. Therapeutic effects of stronger neo-minophagen C at different doses on chronic hepatitis and liver cirrhosis. Hepatol Res. 2001. 19:31–40.9. Yoshida T, Abe K, Ikeda T, Matsushita T, Wake K, Sato T, Inoue H. Inhibitory effect of glycyrrhizin on lipopolysaccharide and d-galactosamine-induced mouse liver injury. Eur J Pharmacol. 2007. 576:136–142.10. Nagai T, Egashira T, Kudo Y, Yamanaka Y, Shimada T. Attenuation of dysfunction in the ischemia-reperfused liver by glycyrrhizin. Jpn J Pharmacol. 1992. 58:209–218.11. Kim YJ, Lee CS. Glycyrrhizin attenuates MPTP neurotoxicity in mouse and MPP-induced cell death in PC12 Cells. Korean J Physiol Pharmacol. 2008. 12:65–71.12. Hwang IK, Lim SS, Choi KH, Yoo KY, Shin HK, Kim EJ, Yoon-Park JH, Kang TC, Kim YS, Kwon DY, Kim DW, Moon WK, Won MH. Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacol Sin. 2006. 27:959–965.13. Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006. 26:6413–6421.14. Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001. 32:2682–2688.15. Lee JK, Hwang WS, Lee YD, Han PL. Dynamic expression of SEK1 suggests multiple roles of the gene during embryogenesis and in adult brain of mice. Brain Res Mol Brain Res. 1999. 66:133–140.16. Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996. 224:855–862.17. Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007. 27:2596–2605.18. Kumada H. Long-term treatment of chronic hepatitis C with glycyrrhizin [stronger neo-minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology. 2002. 62:Suppl 1. 94–100.19. Nakamura T, Fujii T, Ichihara A. Enzyme leakage due to change of membrane permeability of primary cultured rat hepatocytes treated with various hepatotoxins and its prevention by glycyrrhizin. Cell Biol Toxicol. 1985. 1:285–295.20. Shiki Y, Shirai K, Saito Y, Yoshida S, Mori Y, Wakashin M. Effect of glycyrrhizin on lysis of hepatocyte membranes induced by anti-liver cell membrane antibody. J Gastroenterol Hepatol. 1992. 7:12–16.21. Park HY, Park SH, Yoon HK, Han MJ, Kim DH. Anti-allergic activity of 18beta-glycyrrhetinic acid-3-O-beta-D-glucuronide. Arch Pharm Res. 2004. 27:57–60.22. Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000. 120:849–862.23. Ohtsuki K, Abe Y, Shimoyama Y, Furuya T, Munakata H, Takasaki C. Separation of phospholipase A2 in Habu snake venom by glycyrrhizin (GL)-affinity column chromatography and identification of a GL-sensitive enzyme. Biol Pharm Bull. 1998. 21:574–578.24. Kimura M, Inoue H, Hirabayashi K, Natsume H, Ogihara M. Glycyrrhizin and some analogues induce growth of primary cultured adult rat hepatocytes via epidermal growth factor receptors. Eur J Pharmacol. 2001. 431:151–161.25. Yokozawa T, Liu ZW, Chen CP. Protective effects of Glycyrrhizae radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine. 2000. 6:439–445.26. Monder C, Stewart PM, Lakshmi V, Valentino R, Burt D, Edwards CR. Licorice inhibits corticosteroid 11 beta-dehydrogenase of rat kidney and liver: in vivo and in vitro studies. Endocrinology. 1989. 125:1046–1053.27. Cherng JM, Lin HJ, Hung MS, Lin YR, Chan MH, Lin JC. Inhibition of nuclear factor kappaB is associated with neuroprotective effects of glycyrrhizic acid on glutamate-induced excitotoxicity in primary neurons. Eur J Pharmacol. 2006. 547:10–21.28. Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007. 14:431–441.29. Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007. 220:35–46.30. Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008. 28:927–938.31. Ohnishi M, Katsuki H, Fukutomi C, Takahashi M, Motomura M, Fukunaga M, Matsuoka Y, Isohama Y, Izumi Y, Kume T, Inoue A, Akaike A. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011. 61:975–980.32. Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J Pharmacol Exp Ther. 2011. 339:93–98.33. Kim SW, Jin YC, Shin JH, Kim ID, Park S, Han PL, Lee JK. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol Dis. (in press).34. Sakata M, Kawaguchi T, Taniguchi E, Abe M, Koga H, Sata M. Quick and simple method for increasing the reduced albumin fraction in human serum albumin preparations by using stronger neo-minophagen C. Hepatol Res. 2011. 41:1120–1125.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glycyrrhizin Attenuates Kainic Acid-Induced Neuronal Cell Death in the Mouse Hippocampus
- Glycyrrhizin Attenuates MPTP Neurotoxicity in Mouse and MPP+-Induced Cell Death in PC12 Cells
- Effect of Mannitol and Betamethasone on Postischemic Brain Edema in Rabbits
- Inhibition of Oxidative Tissue Damage and Mitochondrial Dysfunction by Glycyrrhizin in the 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Mouse Model of Parkinson's Disease
- Inhibition of LPA5 Activity Provides Long-Term Neuroprotection in Mice with Brain Ischemic Stroke