J Korean Surg Soc.
2011 Oct;81(4):229-234. 10.4174/jkss.2011.81.4.229.
Effect of hypertonic saline and macrophage migration inhibitory factor in restoration of T cell dysfunction
- Affiliations
-
- 1Department of Emergency Medicine, Korea University College of Medicine, Seoul, Korea. kuedchoi@korea.ac.kr
- 2Department of Emergency Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Division of Trauma and Surgical Critical Care and Burns, Department of Surgery, University of California San Diego School of Medicine, San Diego, CA, USA.
- KMID: 1445742
- DOI: http://doi.org/10.4174/jkss.2011.81.4.229
Abstract
- PURPOSE
Trauma-induced suppression of cellular immune function likely contributes to sepsis, multiple organ dysfunction syndrome and death. T cell proliferation decreases after traumatic stress. The addition of prostaglandin E2 (PGE2), which depresses immune function after hemorrhage and trauma, to T-cells decreases T-cell proliferation; and hypertonic saline restores PGE2-induced T-cell suppression. Recently, it has become apparent that macrophage migration inhibitory factor (MIF) plays a central role in several immune responses, including T-cell proliferation. However, the role of MIF in mediating hypertonic saline (HTS) restoration of T cell dysfunction is unknown. Therefore, we hypothesize that T cell immune restoration by HTS occurs, at least in part, by a MIF-mediated mechanism.
METHODS
Jurkat cells were cultured in Roswell Park Memorial Institute media, at a final concentration of 2.5 x 106 cell/mL. The effects of HTS on T-cell proliferation following PGE2-induced suppression were evaluated in Jurkat cells: HTS at 20 or 40 mmol/L above isotonicity was added. MIF levels were determined by enzyme-linked immunosorbent assay and western blot analysis.
RESULTS
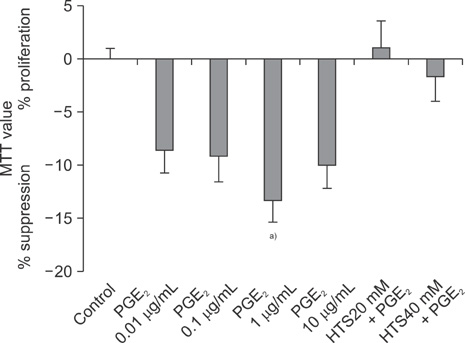
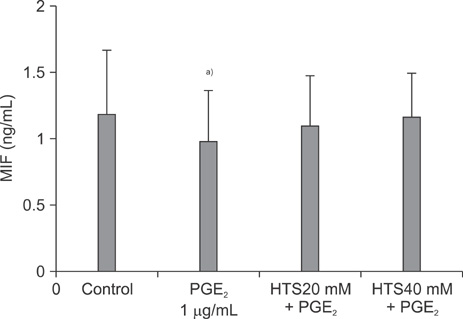
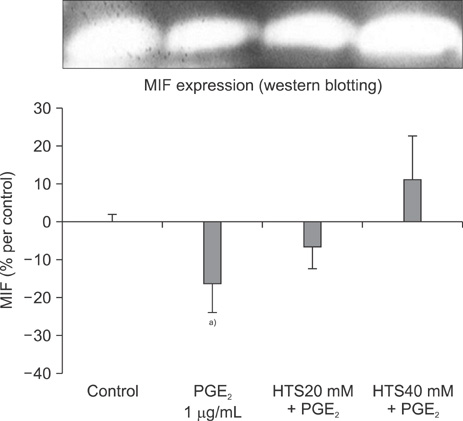
PGE2 caused a 15.0% inhibition of Jurkat cell proliferation, as compared to the control. MIF levels decreased in PGE2-suppressed cells, as compared to the control. MIF levels were higher in cells treated with HTS than PGE2-stimulated cells.
CONCLUSION
The role of HTS in restoring Jurkat cells proliferation suppressed by PGE2, at least in part, should be mediated through a MIF pathway.
Keyword
MeSH Terms
-
Blotting, Western
Cell Proliferation
Dinoprostone
Enzyme-Linked Immunosorbent Assay
Hemorrhage
Humans
Hypertonic Solutions
Jurkat Cells
Macrophage Migration-Inhibitory Factors
Macrophages
Multiple Organ Failure
Negotiating
Prostaglandins E
Sepsis
T-Lymphocytes
Dinoprostone
Hypertonic Solutions
Macrophage Migration-Inhibitory Factors
Prostaglandins E
Figure
Reference
-
1. Napolitano LM, Faist E, Wichmann MW, Coimbra R. Immune dysfunction in trauma. Surg Clin North Am. 1999. 79:1385–1416.2. Coimbra R, Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic/hyperoncotic fluids reverse prostaglandin E2(PGE2)-induced T-cell suppression. Shock. 1995. 4:45–49.3. Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996. 93:7849–7854.4. Bernhagen J, Calandra T, Bucala R. Regulation of the immune response by macrophage migration inhibitory factor: biological and structural features. J Mol Med (Berl). 1998. 76:151–161.5. Lee JY, Song SU, Shin SH. Normal serum macrophage migration inhibitory factor concentrations of Korean people according to age. J Korean Geriatr Soc. 2004. 8:165–169.6. Lehmann LE, Novender U, Schroeder S, Pietsch T, von Spiegel T, Putensen C, et al. Plasma levels of macrophage migration inhibitory factor are elevated in patients with severe sepsis. Intensive Care Med. 2001. 27:1412–1415.7. Lehmann LE, Weber SU, Fuchs D, Klaschik S, Schewe JC, Book M, et al. Intracellular detection of macrophage migration inhibitory factor in peripheral blood leukocytes. Free Radic Biol Med. 2005. 38:1170–1179.8. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.9. Smith JW, Gamelli RL, Jones SB, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med. 2006. 21:160–172.10. Lee JS, Han C, Cho SN, Chung SY. Effect of rapamycin on the cell cycle arrest of T lymphocytes. J Korean Surg Soc. 2007. 73:13–20.11. Park MH, Lee CS, Cho MH, Han C, Koh YS, Kim JC, et al. In vitro induction of carcinoembryonic antigen (CEA) specific cytotoxic T lymphocytes using dendritic cells pulsed with CEA peptide. J Korean Surg Soc. 2005. 69:359–366.12. Santoli D, Phillips PD, Colt TL, Zurier RB. Suppression of interleukin 2-dependent human T cell growth in vitro by prostaglandin E (PGE) and their precursor fatty acids. Evidence for a PGE-independent mechanism of inhibition by the fatty acids. J Clin Invest. 1990. 85:424–432.13. Roper RL, Phipps RP. Prostaglandin E2 regulation of the immune response. Adv Prostaglandin Thromboxane Leukot Res. 1994. 22:101–111.14. Anastassiou ED, Paliogianni F, Balow JP, Yamada H, Boumpas DT. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R alpha gene expression at multiple levels. J Immunol. 1992. 148:2845–2852.15. van Oirschot BA, Stahl M, Lens SM, Medema RH. Protein kinase A regulates expression of p27(kip1) and cyclin D3 to suppress proliferation of leukemic T cell lines. J Biol Chem. 2001. 276:33854–33860.16. Hoyt DB, Junger WG, Loomis WH, Liu FC. Effects of trauma on immune cell function: impairment of intracellular calcium signaling. Shock. 1994. 2:23–28.17. Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic saline enhances cellular immune function. Circ Shock. 1994. 42:190–196.18. Choi SH, Bansal V, Costantini T, Putnam J, Loomis W, Coimbra R. Arginine is essential in reversing prostaglandin E(2) T-cell suppression by hypertonic saline. J Surg Res. 2009. 156:83–89.19. Larson DF, Horak K. Macrophage migration inhibitory factor: controller of systemic inflammation. Crit Care. 2006. 10:138.20. Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002. 4:449–460.21. Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A. 2002. 99:345–350.22. Nguyen MT, Lue H, Kleemann R, Thiele M, Tolle G, Finkelmeier D, et al. The cytokine macrophage migration inhibitory factor reduces pro-oxidative stress-induced apoptosis. J Immunol. 2003. 170:3337–3347.23. Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000. 408:211–216.24. Mitchell RA, Bucala R. Tumor growth-promoting properties of macrophage migration inhibitory factor (MIF). Semin Cancer Biol. 2000. 10:359–366.25. Cho ML, Moon YM, Heo YJ, Woo YJ, Ju JH, Park KS, et al. NF-kappaB inhibition leads to increased synthesis and secretion of MIF in human CD4+ T cells. Immunol Lett. 2009. 123:21–30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypertonic saline downregulate the production level of lipopolysaccharide-induced migration inhibitory factor in THP-1 cells
- The Effect of Subconjunctival Injection of Hypertonic Saline Solution
- Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis
- Normal Serum Macrophage Migration Inhibitory Factor Concentrations of Korean people According to Age
- Expression of Macrophage Migration Inhibitory Factor in Behcet's Disease