J Korean Surg Soc.
2012 Jan;82(1):1-7. 10.4174/jkss.2012.82.1.1.
Hypertonic saline downregulate the production level of lipopolysaccharide-induced migration inhibitory factor in THP-1 cells
- Affiliations
-
- 1Department of Emergency Medicine, Ewha Womans University Hospital, Seoul, Korea.
- 2Department of Emergency Medicine, The Institute for Trauma Research, Korea University College of Medicine, Seoul, Korea. kuedchoi@korea.ac.kr
- KMID: 1437748
- DOI: http://doi.org/10.4174/jkss.2012.82.1.1
Abstract
- PURPOSE
Macrophage migration inhibitory factor (MIF) may serve as a general marker for systemic inflammation in septic and nonseptic acute critical illness. Additionally, our previous experiment has demonstrated that immunosuppressant Prostaglandin E2 (PGE2) lowered MIF levels and inhibited T-cells proliferation when compared to control levels. The addition of hypertonic saline (HTS) increased MIF production as compared with PGE2-stimulated T-cells in concordance with restore PGE2-suppressed T-cells proliferation. Generally, HTS has been well known for its anti-inflammatory effect so far. Therefore, the experiments were conducted to evaluate MIF after stimulating lipopolysaccharide (LPS) either in the presence or absence of HTS in monocyte, in response to early phase injury.
METHODS
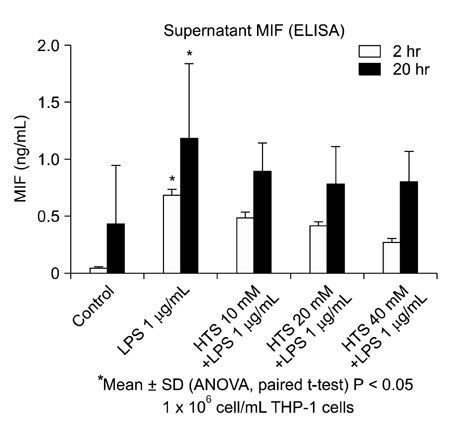
Human acute monocytic leukemic cell line (THP-1) cells were cultured in RPMI media, to a final concentration of 1 x 10(6) cells/mL. The effect of HTS on LPS-induced MIF was evaluated in monocyte with 1 microg/mL LPS. HTS at 10, 20 or 40 mmol/L above isotonicity was added. MIF concentrations of the supernatant were determined by enzyme-linked immunosorbent assay, and cell lysates were used for Western blots analysis to determine the MIF expression.
RESULTS
MIF concentrations in the cell supernatant increased in LPS-induced cells compared to control cells. Also, levels of MIF protein expression were higher in LPS stimulating cells. However, the addition of HTS to LPS stimulated cell restored MIF concentrations and MIF expression.
CONCLUSION
The role of HTS in maintaining physiological balance in human beings, at least in part, should be mediated through the MIF pathway.
Keyword
MeSH Terms
-
Anti-Inflammatory Agents
Blotting, Western
Cell Line
Critical Illness
Dinoprostone
Enzyme-Linked Immunosorbent Assay
Humans
Immunosuppression
Inflammation
Lipopolysaccharides
Macrophage Migration-Inhibitory Factors
Macrophages
Monocytes
Saline Solution, Hypertonic
T-Lymphocytes
Anti-Inflammatory Agents
Dinoprostone
Lipopolysaccharides
Macrophage Migration-Inhibitory Factors
Saline Solution, Hypertonic
Figure
Reference
-
1. Smith JW, Gamelli RL, Jones SB, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med. 2006. 21:160–172.2. Lehmann LE, Weber SU, Fuchs D, Klaschik S, Schewe JC, Book M, et al. Intracellular detection of macrophage migration inhibitory factor in peripheral blood leukocytes. Free Radic Biol Med. 2005. 38:1170–1179.3. Bernhagen J, Calandra T, Bucala R. Regulation of the immune response by macrophage migration inhibitory factor: biological and structural features. J Mol Med (Berl). 1998. 76:151–161.4. Lehmann LE, Novender U, Schroeder S, Pietsch T, von Spiegel T, Putensen C, et al. Plasma levels of macrophage migration inhibitory factor are elevated in patients with severe sepsis. Intensive Care Med. 2001. 27:1412–1415.5. Larson DF, Horak K. Macrophage migration inhibitory factor: controller of systemic inflammation. Crit Care. 2006. 10:138.6. Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002. 4:449–460.7. Schmidt-Supprian M, Murphy C, While B, Lawler M, Kapurniotu A, Voelter W, et al. Activated protein C inhibits tumor necrosis factor and macrophage migration inhibitory factor production in monocytes. Eur Cytokine Netw. 2000. 11:407–413.8. Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996. 93:7849–7854.9. Choi SH, Bansal V, Costantini T, Putnam J, Loomis W, Coimbra R. Arginine is essential in reversing prostaglandin E(2) T-cell suppression by hypertonic saline. J Surg Res. 2009. 156:83–89.10. Coimbra R, Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic/hyperoncotic fluids reverse prostaglandin E2 (PGE2)-induced T-cell suppression. Shock. 1995. 4:45–49.11. Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic saline enhances cellular immune function. Circ Shock. 1994. 42:190–196.12. Saad B, Dakwar S, Said O, Abu-Hijleh G, Al Battah F, Kmeel A, et al. Evaluation of medicinal plant hepatotoxicity in co-cultures of hepatocytes and monocytes. Evid Based Complement Alternat Med. 2006. 3:93–98.13. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.14. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002. 420:860–867.15. Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides. 2003. 37:355–361.16. Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011. 253:431–441.17. Costantini TW, Deree J, Martins JO, Putnam JG, de Campos T, Coimbra R. A novel fluid resuscitation strategy modulates pulmonary transcription factor activation in a murine model of hemorrhagic shock. Clinics (Sao Paulo). 2010. 65:621–628.18. Choi SH, Lee SW, Hong YS, Jeun JM, Min BW. Selective inhibition of polymorphonuclear neutrophils by resuscitative concentration of hypertonic saline. Emerg Med J. 2006. 23:119–122.19. Coimbra R, Hoyt DB, Junger WG, Angle N, Wolf P, Loomis W, et al. Hypertonic saline resuscitation decreases susceptibility to sepsis after hemorrhagic shock. J Trauma. 1997. 42:602–606.20. Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A. 2002. 99:345–350.21. Nguyen MT, Lue H, Kleemann R, Thiele M, Tolle G, Finkelmeier D, et al. The cytokine macrophage migration inhibitory factor reduces pro-oxidative stress-induced apoptosis. J Immunol. 2003. 170:3337–3347.22. Park JK, Kim JK. Influence of MIP-1 Alpha on the CD4+ Th Lymphocytes. J Korean Surg Soc. 2004. 66:81–88.23. Hoyt DB, Junger WG, Loomis WH, Liu FC. Effects of trauma on immune cell function: impairment of intracellular calcium signaling. Shock. 1994. 2:23–28.24. Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004. 3:401–416.25. Chen CF, Cheng CH. Regulation of cellular metabolism and cytokines by the medicinal herb feverfew in the human monocytic THP-1 cells. Evid Based Complement Alternat Med. 2009. 6:91–98.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of hypertonic saline and macrophage migration inhibitory factor in restoration of T cell dysfunction
- Leptin potentiates Prevotella intermedia lipopolysaccharide-induced production of TNF-alpha in monocyte-derived macrophages
- Suppressed Production of Pro-inflammatory Cytokines by LPS-Activated Macrophages after Treatment with Toxoplasma gondii Lysate
- Inhibition of Lipopolysaccharide-stimulated Inflammatory Cytokine Production by LY303511 in Human Macrophagic THP-1 Cells
- Interleukin-8 production and interleukin-8 mRNA expression induced by lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens in monocyte-derived macrophages