Lab Anim Res.
2012 Sep;28(3):165-170. 10.5625/lar.2012.28.3.165.
Comparison of alpha-synuclein immunoreactivity in the spinal cord between the adult and aged beagle dog
- Affiliations
-
- 1Laboratory of Neuroscience, Department of Physical Therapy, College of Rehabilitation Science, Daegu University, Gyeongsan, Korea.
- 2Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea. mhwon@kangwon.ac.kr
- 3Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon, Korea.
- 4Department of Physiology, College of Medicine, and Institute of Neurodegeneration and Neuroregeneration, Hallym University, Chuncheon, Korea. hcshin@hallym.ac.kr
- KMID: 1436721
- DOI: http://doi.org/10.5625/lar.2012.28.3.165
Abstract
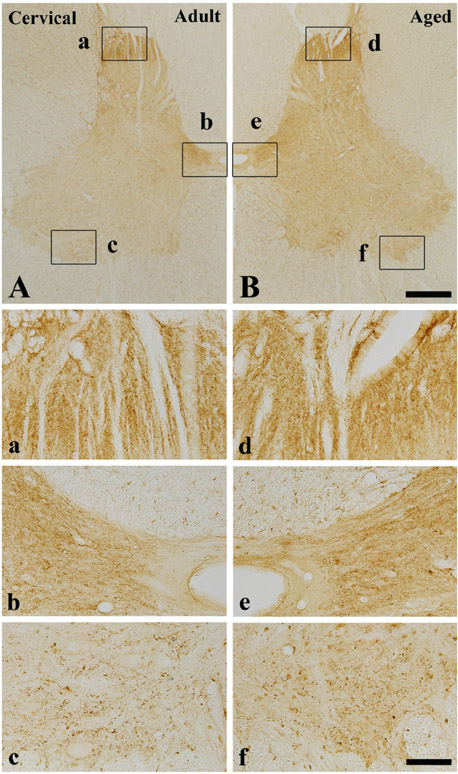
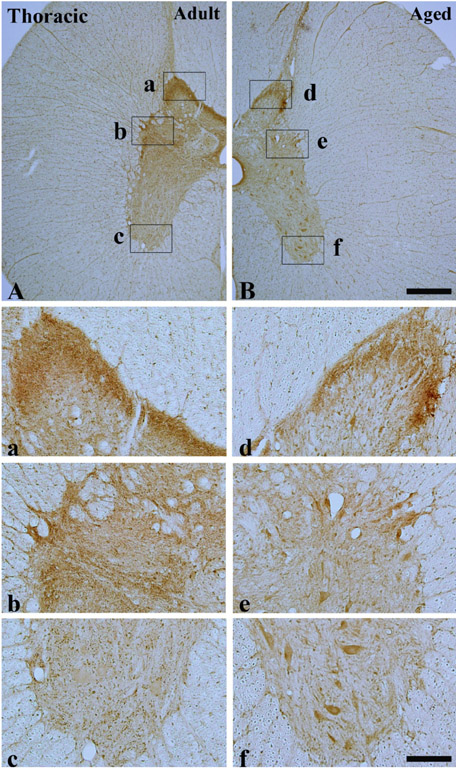
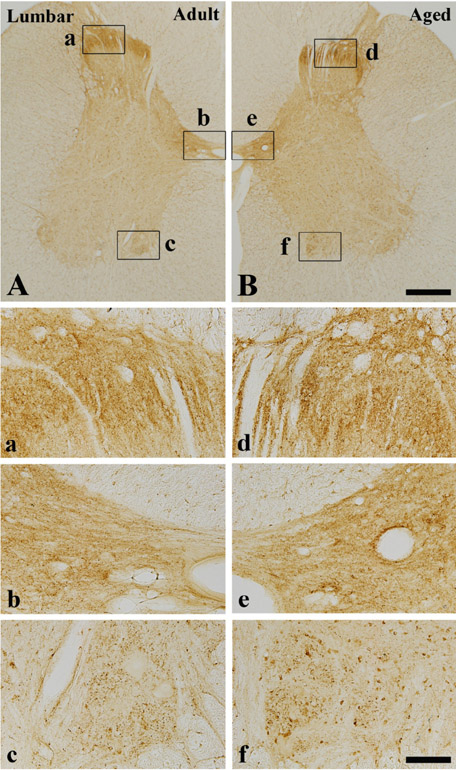
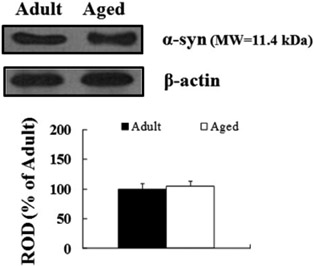
- Alpha-synuclein (alpha-syn) is a presynaptic protein that is richly expressed in the central and peripheral nervous systems of mammals, and it is related to the pathogenesis of Parkinson's disease and other neurodegenerative disorders. In the present study, we compared the distribution of the immunoreactivity of alpha-syn and its related gliosis in the spinal cord of young adult (2-3 years) and aged (10-12 years) beagle dogs. We discovered that alpha-syn immunoreactivity was present in many neurons in the thoracic level of the aged spinal cord, however, its protein level was not distinct inform that of the adult spinal cord. In addition, ionized calcium-binding adapter molecule-1 (a marker for microglia) immunoreactivity, and not glial fibrillary acidic protein (a marker for astrocytes) immunoreactivity, was somewhat increased in the aged group compared to the adult group. These results indicate that alpha-syn immunoreactivity was not dramatically changed in the dog spinal cord during aging.
Keyword
MeSH Terms
Figure
Reference
-
1. Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000. 25(1):239–252.2. Totterdell S, Meredith GE. Localization of alpha-synuclein to identified fibers and synapses in the normal mouse brain. Neuroscience. 2005. 135(3):907–913.3. Li JY, Henning Jensen P, Dahlström A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002. 113(2):463–478.4. Irizarry MC, Kim TW, McNamara M, Tanzi RE, George JM, Clayton DF, Hyman BT. Characterization of the precursor protein of the non-A beta component of senile plaques (NACP) in the human central nervous system. J Neuropathol Exp Neurol. 1996. 55(8):889–895.5. Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha - synuclein in human and transgenic mouse brain. J Neurosci. 2000. 20(17):6365–6373.6. Trojanowski JQ, Lee VM. Aggregation of neurofilament and alpha-synuclein proteins in Lewy bodies: implications for the pathogenesis of Parkinson disease and Lewy body dementia. Arch Neurol. 1998. 55(2):151–152.7. Eller M, Williams DR. α-Synuclein in Parkinson disease and other neurodegenerative disorders. Clin Chem Lab Med. 2011. 49(3):403–408.8. Rampello L, Cerasa S, Alvano A, Buttà V, Raffaele R, Vecchio I, Cavallaro T, Cimino E, Incognito T, Nicoletti F. Dementia with Lewy bodies: a review. Arch Gerontol Geriatr. 2004. 39(1):1–14.9. Seilhean D, Takahashi J, El Hachimi KH, Fujigasaki H, Lebre AS, Biancalana V, Dürr A, Salachas F, Hogenhuis J, de Thé H, Hauw JJ, Meininger V, Brice A, Duyckaerts C. Amyotrophic lateral sclerosis with neuronal intranuclear protein inclusions. Acta Neuropathol. 2004. 108(1):81–87.10. Wenning GK, Jellinger KA. The role of alpha-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol. 2005. 109(2):129–140.11. Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Sisk A, Masliah E. Abnormal distribution of the non-Abeta component of Alzheimer's disease amyloid precursor/alpha-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. Lab Invest. 1998. 78(9):1169–1177.12. Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998. 249(2-3):180–182.13. Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/-) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A. 2001. 98(5):2278–2283.14. Ahn JH, Choi JH, Kim JS, Lee HJ, Lee CH, Yoo KY, Hwang IK, Lee YL, Shin HC, Won MH. Comparison of immunoreactivities in 4-HNE and superoxide dismutases in the cervical and the lumbar spinal cord between adult and aged dogs. Exp Gerontol. 2011. 46(8):703–708.15. Monti B, Contestabile A. Selective alteration of DNA fragmentation and caspase activity in the spinal cord of aged rats and effect of dietary restriction. Brain Res. 2003. 992(1):137–141.16. Sotiriou E, Vassilatis DK, Vila M, Stefanis L. Selective noradrenergic vulnerability in α-synuclein transgenic mice. Neurobiol Aging. 2010. 31(12):2103–2114.17. Vivacqua G, Yin JJ, Casini A, Li X, Li YH, D'Este L, Chan P, Renda TG, Yu S. Immunolocalization of alpha-synuclein in the rat spinal cord by two novel monoclonal antibodies. Neuroscience. 2009. 158(4):1478–1487.18. Chung YH, Joo KM, Kim MJ, Cha CI. Immunohistochemical study on the distribution of alpha-synuclein in the central nervous system of transgenic mice expressing a human Cu/Zn superoxide dismutase mutation. Neurosci Lett. 2003. 342(3):151–154.19. Vivacqua G, Casini A, Vaccaro R, Fornai F, Yu S, D'Este L. Different sub-cellular localization of alpha-synuclein in the C57BL\6J mouse's central nervous system by two novel monoclonal antibodies. J Chem Neuroanat. 2011. 41(2):97–110.20. Oinas M, Paetau A, Myllykangas L, Notkola IL, Kalimo H, Polvikoski T. alpha-Synuclein pathology in the spinal cord autonomic nuclei associates with alpha-synuclein pathology in the brain: a population-based Vantaa 85+ study. Acta Neuropathol. 2010. 119(6):715–722.21. Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006. 32(3):284–295.22. Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006. 66(7):1100–1102.23. Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992. 587(2):250–256.24. Horner PJ, Palmer TD. New roles for astrocytes: the nightlife of an 'astrocyte'. La vida loca! Trends Neurosci. 2003. 26(11):597–603.25. Vaca K, Wendt E. Divergent effects of astroglial and microglial secretions on neuron growth and survival. Exp Neurol. 1992. 118(1):62–72.26. Croisier E, Graeber MB. Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol. 2006. 112(5):517–530.27. Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006. 20(12):2000–2008.28. Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010. 285(12):9262–9272.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of arylalkylamine N-acetyltransferase and melatonin receptor type 1B immunoreactivity between young adult and aged canine spinal cord
- Alpha-Synuclein Function and Dysfunction on Cellular Membranes
- Accumulation of Alpha-synuclein Causes Colonic Dysmotility Independently of Enteric Nervous Damage in the Early Stage of Parkinson's Disease (Neurogastroenterol Motil 2012;24:e425-e436)
- Identification and Localization of Alpha-Synuclein in Human Cornea
- Structure, Distribution, and Genetic Profile of α-Synuclein and Their Potential Clinical Application in Parkinson's Disease