Korean J Physiol Pharmacol.
2013 Feb;17(1):51-56. 10.4196/kjpp.2013.17.1.51.
Phorbol 12-Myristate 13-Acetate Enhances Long-Term Potentiation in the Hippocampus through Activation of Protein Kinase Cdelta and epsilon
- Affiliations
-
- 1Department of Physiology and Biophysics, School of Medicine, Eulji University, Daejeon 301-746, Korea. ssmin@eulji.ac.kr
- 2Department of Anesthesiology and Pain Medicine, School of Medicine, Konkuk University, Chungju 380-701, Korea.
- 3Department of Basic Nursing Science, School of Nursing, Korea University, Seoul 136-703, Korea.
- KMID: 1432763
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.51
Abstract
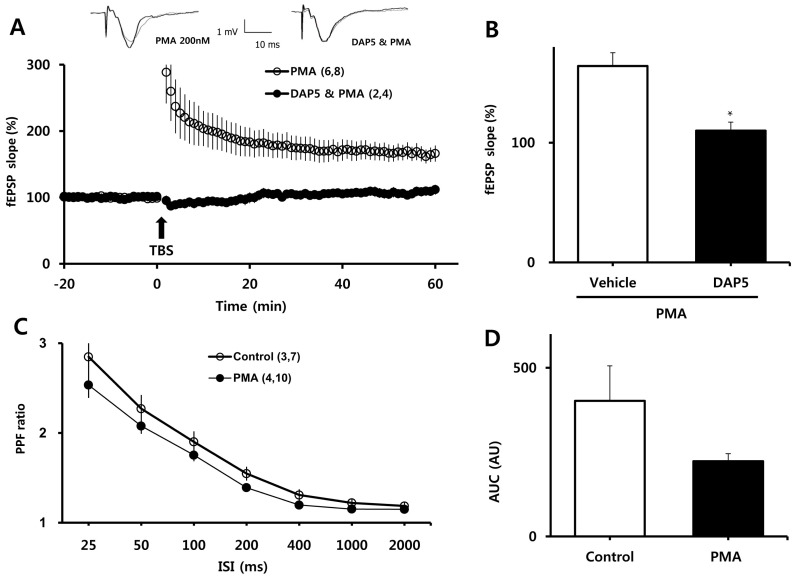
- Many intracellular proteins and signaling cascades contribute to the sensitivity of N-methyl-D-aspartate receptors (NMDARs). One such putative contributor is the serine/threonine kinase, protein kinase C (PKC). Activation of PKC by phorbol 12-myristate 13-acetate (PMA) causes activation of extracellular signal-regulated kinase (ERK) and promotes the formation of new spines in cultured hippocampal neurons. The purpose of this study was to examine which PKC isoforms are responsible for the PMA-induced augmentation of long-term potentiation (LTP) in the CA1 stratum radiatum of the hippocampus in vitro and verify that this facilitation requires NMDAR activation. We found that PMA enhanced the induction of LTP by a single episode of theta-burst stimulation in a concentration-dependent manner without affecting to magnitude of baseline field excitatory postsynaptic potentials. Facilitation of LTP by PMA (200 nM) was blocked by the nonspecific PKC inhibitor, Ro 31-8220 (10microM); the selective PKCdelta inhibitor, rottlerin (1microM); and the PKCepsilon inhibitor, TAT-epsilonV1-2 peptide (500 nM). Moreover, the NMDAR blocker DL-APV (50microM) prevented enhancement of LTP by PMA. Our results suggest that PMA contributes to synaptic plasticity in the nervous system via activation of PKCdelta and/or PKCepsilon, and confirm that NMDAR activity is required for this effect.
Keyword
MeSH Terms
-
2-Amino-5-phosphonovalerate
Acetophenones
Benzopyrans
Excitatory Postsynaptic Potentials
Hippocampus
Indoles
Long-Term Potentiation
Nervous System
Neurons
Phorbols
Phosphotransferases
Protein Isoforms
Protein Kinases
Proteins
Receptors, N-Methyl-D-Aspartate
Spine
2-Amino-5-phosphonovalerate
Acetophenones
Benzopyrans
Indoles
Phorbols
Phosphotransferases
Protein Isoforms
Protein Kinases
Proteins
Receptors, N-Methyl-D-Aspartate
Figure
Cited by 2 articles
-
Effect of Phorbol 12-Myristate 13-Acetate on the Differentiation of Adipose-Derived Stromal Cells from Different Subcutaneous Adipose Tissue Depots
Jennifer K. Song, Chang Hoon Lee, So-Min Hwang, Bo Sun Joo, Sun Young Lee, Jin Sup Jung
Korean J Physiol Pharmacol. 2014;18(4):289-296. doi: 10.4196/kjpp.2014.18.4.289.Long-term Synaptic Plasticity: Circuit Perturbation and Stabilization
Joo Min Park, Sung-Cherl Jung, Su-Yong Eun
Korean J Physiol Pharmacol. 2014;18(6):457-460. doi: 10.4196/kjpp.2014.18.6.457.
Reference
-
1. Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993; 361:31–39. PMID: 8421494.
Article2. Gustafsson B, Wigström H. Long-term potentiation in the hippocampal CA1 region: its induction and early temporal development. Prog Brain Res. 1990; 83:223–232. PMID: 2203099.3. Izquierdo I, Cammarota M, Da Silva WC, Bevilaqua LR, Rossato JI, Bonini JS, Mello P, Benetti F, Costa JC, Medina JH. The evidence for hippocampal long-term potentiation as a basis of memory for simple tasks. An Acad Bras Cienc. 2008; 80:115–127. PMID: 18345380.
Article4. Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004; 5:952–962. PMID: 15550950.
Article5. Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001; 2:315–324. PMID: 11331915.
Article6. Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001; 101:2353–2364. PMID: 11749377.
Article7. Ohno S, Nishizuka Y. Protein kinase C isotypes and their specific functions: prologue. J Biochem. 2002; 132:509–511. PMID: 12359062.8. Coussens L, Rhee L, Parker PJ, Ullrich A. Alternative splicing increases the diversity of the human protein kinase C family. DNA. 1987; 6:389–394. PMID: 3677994.
Article9. Roisin MP, Barbin G. Differential expression of PKC isoforms in hippocampal neuronal cultures: modifications after basic FGF treatment. Neurochem Int. 1997; 30:261–270. PMID: 9041557.
Article10. Tanaka C, Saito N, Kose A, Ito A, Hosoda K, Sakaue M, Shuntoh H, Nishino N, Taniyama K. Possible roles of protein kinase C in neurotransmission. Adv Exp Med Biol. 1988; 236:277–285. PMID: 3239487.
Article11. Saito N, Shirai Y. Protein kinase C gamma (PKC gamma): function of neuron specific isotype. J Biochem. 2002; 132:683–687. PMID: 12417016.12. Swope SL, Moss SJ, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res. 1999; 33:49–78. PMID: 10218114.13. Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008; 28:7820–7827. PMID: 18667614.14. Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res. 2008; 169:27–40. PMID: 18394466.15. Rozengurt E. Early signals in the mitogenic response. Science. 1986; 234:161–166. PMID: 3018928.
Article16. Xue R, Zhao Y, Chen P. Involvement of PKC alpha in PMA-induced facilitation of exocytosis and vesicle fusion in PC12 cells. Biochem Biophys Res Commun. 2009; 380:371–376. PMID: 19250646.17. Goldin M, Segal M. Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur J Neurosci. 2003; 17:2529–2539. PMID: 12823460.
Article18. Linden DJ, Routtenberg A. The role of protein kinase C in long-term potentiation: a testable model. Brain Res Brain Res Rev. 1989; 14:279–296. PMID: 2679942.
Article19. Angenstein F, Staak S. Receptor-mediated activation of protein kinase C in hippocampal long-term potentiation: facts, problems and implications. Prog Neuropsychopharmacol Biol Psychiatry. 1997; 21:427–454. PMID: 9153067.
Article20. Van der Zee EA, Luiten PG, Disterhoft JF. Learning-induced alterations in hippocampal PKC-immunoreactivity: a review and hypothesis of its functional significance. Prog Neuropsychopharmacol Biol Psychiatry. 1997; 21:531–572. PMID: 9153071.
Article21. Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 2010; 30:242–254. PMID: 20053906.
Article22. Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001; 4:382–390. PMID: 11276228.
Article23. Yan JZ, Xu Z, Ren SQ, Hu B, Yao W, Wang SH, Liu SY, Lu W. Protein kinase C promotes N-methyl-D-aspartate (NMDA) receptor trafficking by indirectly triggering calcium/calmodulin-dependent protein kinase II (CaMKII) autophosphorylation. J Biol Chem. 2011; 286:25187–25200. PMID: 21606495.
Article24. Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003; 45:755–767. PMID: 14529714.
Article25. Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001; 21:3063–3072. PMID: 11312291.
Article26. Moriguchi S, Shioda N, Han F, Yeh JZ, Narahashi T, Fukunaga K. Galantamine enhancement of long-term potentiation is mediated by calcium/calmodulin-dependent protein kinase II and protein kinase C activation. Hippocampus. 2009; 19:844–854. PMID: 19253410.
Article27. Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997; 276:2042–2045. PMID: 9197267.
Article28. Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998; 279:870–873. PMID: 9452388.29. Sun MK, Alkon DL. Dual effects of bryostatin-1 on spatial memory and depression. Eur J Pharmacol. 2005; 512:43–51. PMID: 15814089.
Article30. Sun MK, Hongpaisan J, Nelson TJ, Alkon DL. Poststroke neuronal rescue and synaptogenesis mediated in vivo by protein kinase C in adult brains. Proc Natl Acad Sci USA. 2008; 105:13620–13625. PMID: 18768786.
Article31. Olds JL, Alkon DL. Protein kinase C: a nexus in the biochemical events that underlie associative learning. Acta Neurobiol Exp (Wars). 1993; 53:197–207. PMID: 8317248.32. Linden DJ, Sheu FS, Murakami K, Routtenberg A. Enhancement of long-term potentiation by cis-unsaturated fatty acid: relation to protein kinase C and phospholipase A2. J Neurosci. 1987; 7:3783–3792. PMID: 2824717.
Article33. Luo Y, Vallano ML. Arachidonic acid, but not sodium nitroprusside, stimulates presynaptic protein kinase C and phosphorylation of GAP-43 in rat hippocampal slices and synaptosomes. J Neurochem. 1995; 64:1808–1818. PMID: 7891109.
Article34. Moriguchi S, Oomura Y, Shioda N, Han F, Hori N, Aou S, Fukunaga K. Ca2+/calmodulin-dependent protein kinase II and protein kinase C activities mediate extracellular glucose-regulated hippocampal synaptic efficacy. Mol Cell Neurosci. 2011; 46:101–107. PMID: 20807573.35. Hongpaisan J, Alkon DL. A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc Natl Acad Sci USA. 2007; 104:19571–19576. PMID: 18073185.
Article36. Swannie HC, Kaye SB. Protein kinase C inhibitors. Curr Oncol Rep. 2002; 4:37–46. PMID: 11734112.
Article37. Bank B, DeWeer A, Kuzirian AM, Rasmussen H, Alkon DL. Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci USA. 1988; 85:1988–1992. PMID: 3162320.
Article38. Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986; 321:175–177. PMID: 3010137.
Article39. Routtenberg A, Colley P, Linden D, Lovinger D, Murakami K, Sheu FS. Phorbol ester promotes growth of synaptic plasticity. Brain Res. 1986; 378:374–378. PMID: 3015357.
Article40. Reneau JC, Reyland ME, Phillips J, Kindy C, Popp RL. Phorbol 12-myristate 13-acetate potentiation of N-methyl-D-aspartateinduced currents in primary cultured cerebellar granule cells is mediated by protein kinase C alpha. J Pharmacol Exp Ther. 2009; 330:641–649. PMID: 19429793.41. Hendey B, Zhu CL, Greenstein S. Fas activation opposes PMA-stimulated changes in the localization of PKCdelta: a mechanism for reducing neutrophil adhesion to endothelial cells. J Leukoc Biol. 2002; 71:863–870. PMID: 11994512.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Activation of Protein Kinase C on the Calcium-dependent K; Current in Rat Basilar Smooth Muscle Cells
- Phorbol Esters Attenuate The Action of Isoproterenol on Vascular Smooth Muscle
- Differential Physiological Effects of Raf-1 Kinase Pathways Linked to Protein Kinase C Activation Depending on the Stimulus in v-H-ras-transformed Cells
- Effect of phorbol ester on K+ channel in an G292 osteoblast-like cell
- Phorbol Ester Modulates th Action of Acetylcholine in Rabbit Carotid Artery