Cancer Res Treat.
2008 Jun;40(2):39-44.
Differential Physiological Effects of Raf-1 Kinase Pathways Linked to Protein Kinase C Activation Depending on the Stimulus in v-H-ras-transformed Cells
- Affiliations
-
- 1Department of Biology, College of Natural Sciences, University of Incheon, Incheon, Korea. mikelee@incheon.ac.kr
Abstract
-
PURPOSE: We investigated the molecular mechanism by which the Raf-1 kinase pathways that are linked to protein kinase C induce differential physiological effects, depending on the stimulus, by employing the pharmacological PKC activator PMA.
MATERIALS AND METHODS
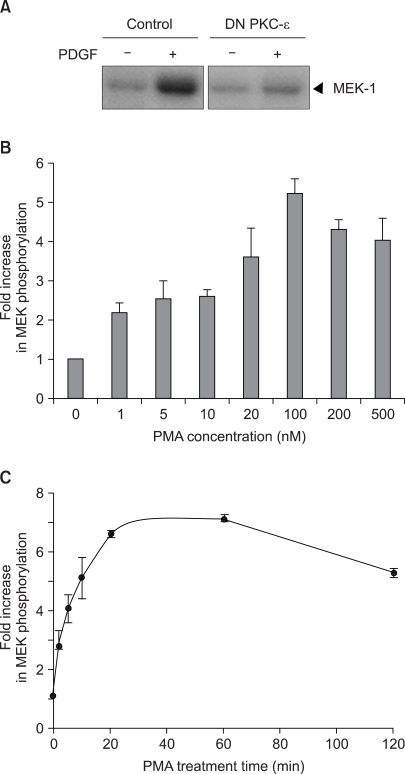
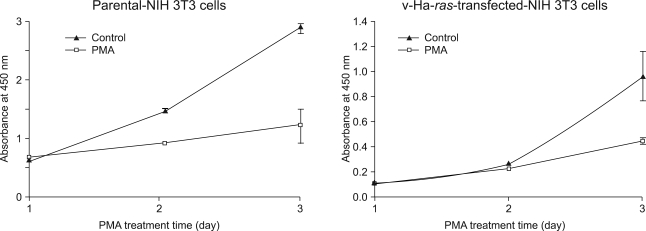
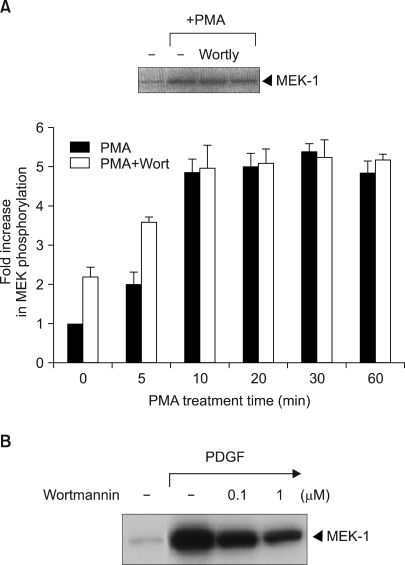
Parental and v-Ha-ras transfected NIH 3T3 cells were chosen as test systems and these cells were transiently transfected with the pMTH vector that encodes dominant-negative (DN) PKC-epsilon with using Lipofectamine 2000. The cell proliferation reagent WST-1 was used for the quantitative determination of cellular proliferation. The Raf-1 kinase activity was measured by assessing the phosphorylation of recombinant MEK with using the immunoprecipitated Raf-1 proteins. The phosphorylated MEK protein bands were quantified by using Quantity One analysis software.
RESULTS
The pharmacological PKC activator phorbol-12-myristate-13-acetate (PMA) and platelet-derived growth factor (PDGF) were able to induce the activation of Raf-1 kinase in the v-H-ras-transformed NIH3T3 fibroblasts. However, PMA was found to be much less sensitive PI3 kinase inhibitor or the chemical antioxidant than is PDGF. Especially, PMA mediated growth arrest while PDGF induced mitogenic signaling through the PKC-epsilon activation. Thus, the regulation of the Raf-1 cascade by both PDGF and PMA is likely to be intimately linked and they converge at the PKC level through different upstream pathways, as was shown by the inhibition of PDGF-induced Raf-1 kinase activation by the transient transfection with a dominant-negative mutant of PKC-epsilon.
CONCLUSIONS
Taken together, these results imply that, depending on the stimulus, Raf-1 kinase leads to different physiological effects.
Keyword
- Raf-1 kinase; PKC; PMA; PDGF; Cell
MeSH Terms
-
Cell Proliferation
Fibroblasts
Humans
Lipids
NIH 3T3 Cells
Parents
Phosphorylation
Phosphotransferases
Platelet-Derived Growth Factor
Protein Kinase C
Protein Kinases
Proteins
Proto-Oncogene Proteins c-raf
Transfection
Lipids
Phosphotransferases
Platelet-Derived Growth Factor
Protein Kinase C
Protein Kinases
Proteins
Proto-Oncogene Proteins c-raf
Figure
Reference
-
1. Rapp UR, Goldsborough MD, Mark GE, Bonner TI, Groffen J, Reynolds FH Jr, et al. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci USA. 1983; 80:4218–4222. PMID: 6308607.
Article2. Morrison DK, Cutler R. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997; 9:174–179. PMID: 9069260.
Article3. Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco MT, Moscat J, et al. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997; 17:732–741. PMID: 9001227.
Article4. Dhillon AS, von Krieqsheim A, Grindlay J, Kolch W. Phosphatase and feedback regulation of Raf-1 signaling. Cell Cycle. 2007; 6:3–7. PMID: 17218791.
Article5. Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem. 2003; 278:13061–13068. PMID: 12551925.
Article6. Nakajima T. Signaling cascades in radiation-induced apoptosis: roles of protein kinase C in the apoptosis regulation. Med Sci Monit. 2006; 12:RA220–RA224. PMID: 17006414.7. Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000; 19:496–503. PMID: 10675318.
Article8. Blobe GC, Stribling S, Obeid LM, Hannun YA. Protein kinase C isoenzymes: regulation and function. Cancer Surv. 1996; 27:213–248. PMID: 8909803.9. Perletti GP, Concari P, Brusaferri S, Marras E, Piccinini F, Tashjian AHJ. Protein kinase C ε is oncogenic in colon epithelial cells by interaction with the ras signal transduction pathway. Oncogene. 1998; 16:3345–3348. PMID: 9681835.10. Mischak H, Goodnight JA, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz MG, et al. Overexpression of protein kinase C-δ and -ε in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993; 268:6090–6096. PMID: 8454583.11. Gomez-Fernandez JC, Torrecillas A, Corbalan-Garcia S. Diacylglycerol as activators of protein kinase C. Mol Membr Biol. 2004; 21:339–349. PMID: 15764364.12. Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984; 225:1365–1370. PMID: 6147898.
Article13. Lee M, Petrovics G, Anderson WB. The synergistic activation of Raf-1 kinase by phorbol myristate acetate and hydrogen peroxide in NIH 3T3 cells. Biochem Biophys Res Commun. 2003; 311:1026–1033. PMID: 14623285.14. Morrison DK, Kaplan DR, Escobedo JA, Rapp UR, Roberts TM, Williams LT. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF b-receptor. Cell. 1989; 58:649–657. PMID: 2475255.15. Li BQ, Subleski M, Shalloway D, Kung HF, Kamata T. Mitogenic activation of the Ras guanine nucleotide exchange factor in NIH 3T3 cells involves protein tyrosine phosphorylation. Proc Natl Acad Sci USA. 1993; 90:8504–8508. PMID: 8104337.
Article16. Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993; 296:297–301. PMID: 8257416.
Article17. Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997; 94:11233–11237. PMID: 9326592.18. Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol. 2000; 72:12–19. PMID: 10979203.19. Wheeler DL, Ness KJ, Oberley TD, Verma AK. Protein kinase c is linked to 12-O-tetradecanoylphorbol-13-acetate-induced tumor necrosis factor-a ectodomain shedding and the development of metastatic squamous cell carcinoma in protein kinase C ε transgenic mice. Cancer Res. 2003; 63:6547–6555. PMID: 14559850.20. Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998; 18:790–798. PMID: 9447975.
Article21. Ueffing M, Lovric J, Philipp A, Mischak H, Kolch W. Protein kinase C-e associates with the Raf-1 kinase and induces the production of growth factors that stimulate Raf-1 activity. Oncogene. 1997; 15:2921–2927. PMID: 9416835.22. Moriya S, Kazlauskas A, Akimoto K, Hirai S, Mizuno K, Takenawa T, et al. Platelet-derived growth factor activates protein kinase C ε through redundant and independent signaling pathways involving phospholipase C γ or phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996; 93:151–155. PMID: 8552594.23. Scheid MP, Duronio V. Phosphatidylinositol 3-OH kinase activity is not required for activation of mitogen-activated protein kinase by cytokines. J Biol Chem. 1996; 271:18134–18139. PMID: 8663437.
Article24. Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995; 270:296–299. PMID: 7569979.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ras Mitogen-activated Protein Kinase Signaling and Kinase Suppressor of Ras as Therapeutic Targets for Hepatocellular Carcinoma
- Molecular assembly of mitogen-activated protein kinase module in ras-transformed NIH3T3 cell line
- The Difference in Biological Properties between Parental and v-Ha-ras Transformed NIH3T3 Cells
- Signal Transduction Mechanisms Mediating Surfactant Phospholipid Secretion in Isolated Type II Cell
- Differential Activation of Ras/Raf/MAPK Pathway between Heart and Cerebral Artery in Isoproterenol-induced Cardiac Hypertrophy