Korean J Physiol Pharmacol.
2013 Apr;17(2):127-132. 10.4196/kjpp.2013.17.2.127.
Inhibitory Effects of Ginsenoside Metabolites, Compound K and Protopanaxatriol, on GABAC Receptor-Mediated Ion Currents
- Affiliations
-
- 1Ginsentology Research Laboratory and Department of Physiology, College of Veterinary Medicine and Bio/Molecular Informatics Center, Konkuk University, Seoul 143-701, Korea. synah@konkuk.ac.kr
- 2Department of Physical Therapy, Sehan University, Yeongam 526-702, Korea.
- 3Seoul Center, Korea Basic Science Institute, Seoul 136-701, Korea.
- KMID: 1429383
- DOI: http://doi.org/10.4196/kjpp.2013.17.2.127
Abstract
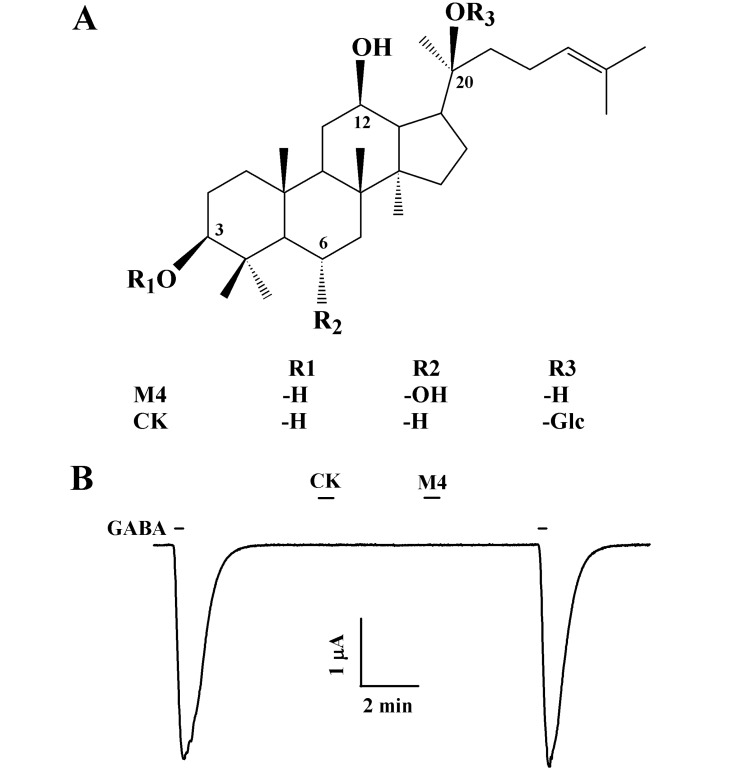
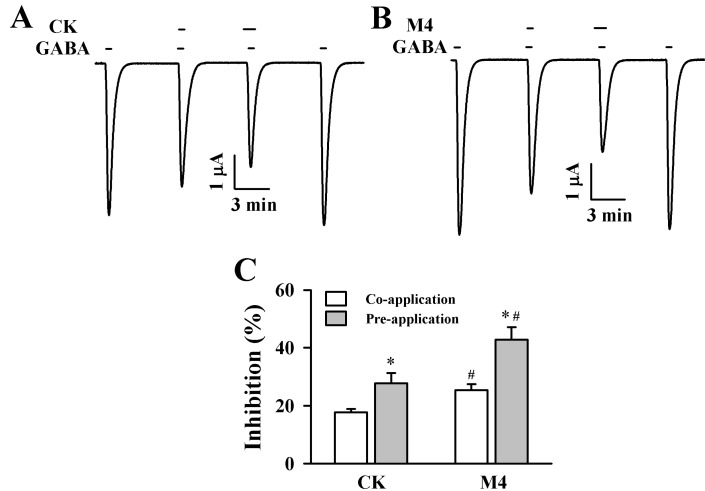
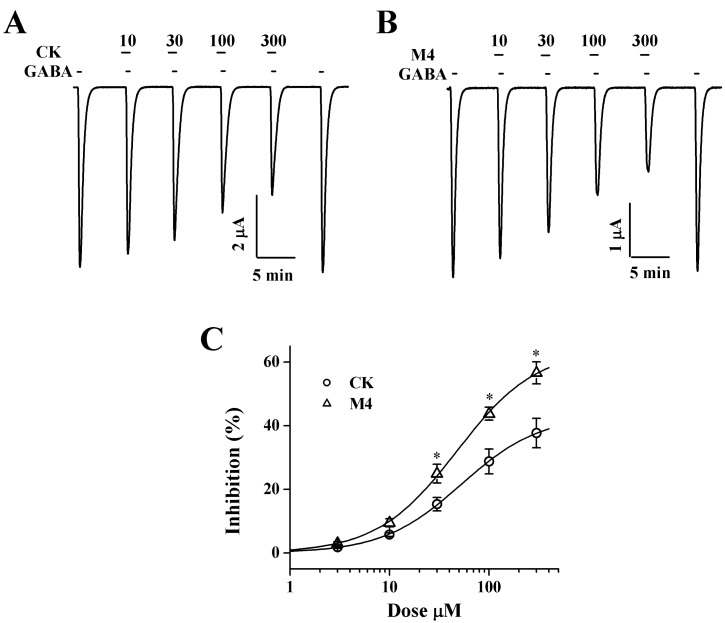
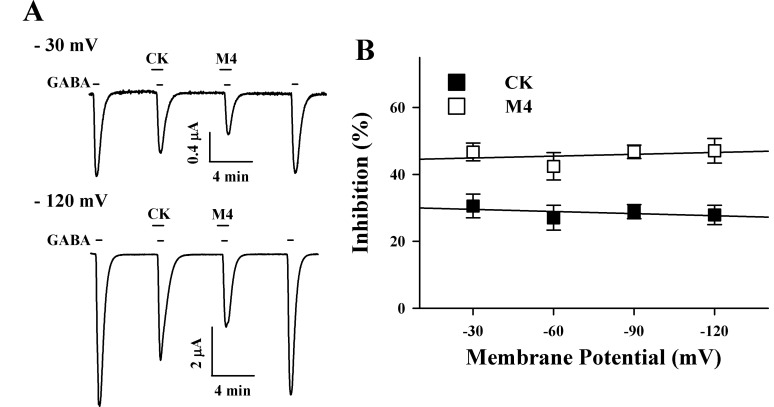
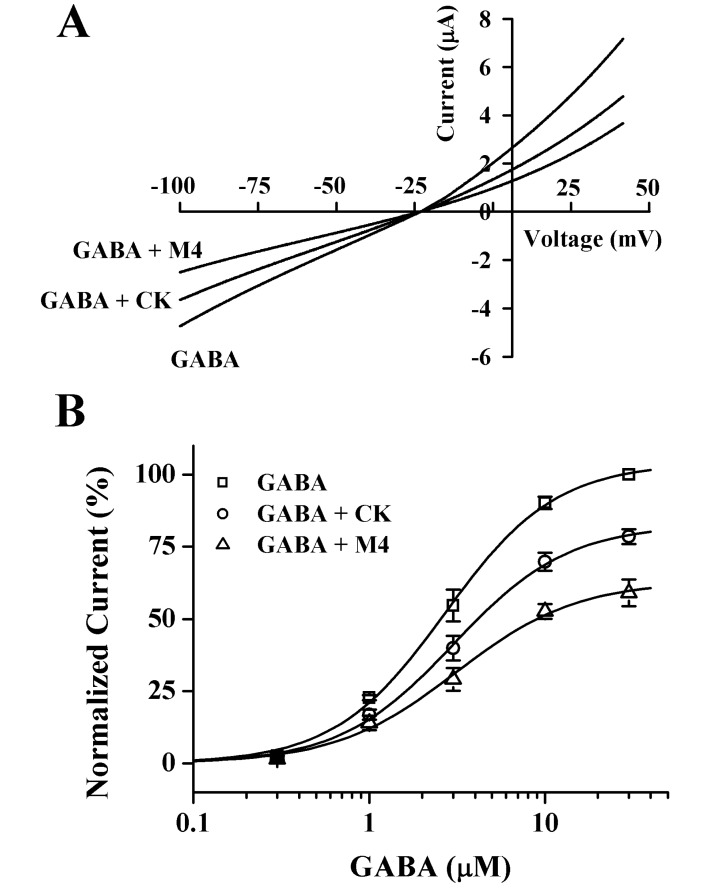
- Ginsenosides, one of the active ingredients of Panax ginseng, show various pharmacological and physiological effects, and they are converted into compound K (CK) or protopanaxatriol (M4) by intestinal microorganisms. CK is a metabolite derived from protopanaxadiol (PD) ginsenosides, whereas M4 is a metabolite derived from protopanaxatriol (PT) ginsenosides. The gamma-aminobutyric acid receptorC (GABAC) is primarily expressed in retinal bipolar cells and several regions of the brain. However, little is known of the effects of ginsenoside metabolites on GABAC receptor channel activity. In the present study, we examined the effects of CK and M4 on the activity of human recombinant GABAC receptor (rho1) channels expressed in Xenopus oocytes by using a 2-electrode voltage clamp technique. In oocytes expressing GABAC receptor cRNA, we found that CK or M4 alone had no effect in oocytes. However, co-application of either CK or M4 with GABA inhibited the GABA-induced inward peak current (IGABA). Interestingly, pre-application of M4 inhibited IGABA more potently than CK in a dose-dependent and reversible manner. The half-inhibitory concentration (IC50) values of CK and M4 were 52.1+/-2.3 and 45.7+/-3.9 microM, respectively. Inhibition of IGABA by CK and M4 was voltage-independent and non-competitive. This study implies that ginsenoside metabolites may regulate GABAC receptor channel activity in the brain, including in the eyes.
MeSH Terms
Figure
Reference
-
1. Nah SY. Ginseng: recent advances and trends. Korean J Ginseng Sci. 1997; 21:1–12.2. Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007; 13:381–404. PMID: 18078425.
Article3. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58:1685–1693. PMID: 10571242.4. Hasegawa H, Sung JH, Matsumiya S, Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996; 62:453–457. PMID: 8923812.
Article5. Kanaoka M, Akao T, Kobashi K. Metabolism of ginseng saponins, ginsenosides by human intestinal flora. J Trad Med. 1994; 11:241–245.6. Karikura M, Miyase T, Tanizawa H, Taniyama T, Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VI. The decomposition products of ginsenoside Rb2 in the stomach of rats. Chem Pharm Bull (Tokyo). 1991; 39:400–404. PMID: 2054864.
Article7. Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002; 25:861–866. PMID: 12132658.
Article8. Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998; 246:725–730. PMID: 9618279.
Article9. Bormann J. The 'ABC' of GABA receptors. Trends Pharmacol Sci. 2000; 21:16–19. PMID: 10637650.
Article10. Chebib M, Johnston GA. GABA-Activated ligand gated ion channels: medicinal chemistry and molecular biology. J Med Chem. 2000; 43:1427–1447. PMID: 10780899.
Article11. Zhang D, Pan ZH, Awobuluyi M, Lipton SA. Structure and function of GABAC receptors: a comparison of native versus recombinant receptors. Trends Pharmacol Sci. 2001; 22:121–132. PMID: 11239575.12. Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998; 38:1411–1430. PMID: 9667008.
Article13. McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the rho1 subunit abolishes GABAC receptor expression and alters visual processing in the mouse retina. J Neurosci. 2002; 22:4163–4174. PMID: 12019334.14. Drew CA, Johnston GA, Weatherby RP. Bicuculline-insensitive GABA receptors: studies on the binding of (-)-baclofen to rat cerebellar membranes. Neurosci Lett. 1984; 52:317–321. PMID: 6097844.
Article15. Strata F, Cherubini E. Transient expression of a novel type of GABA response in rat CA3 hippocampal neurones during development. J Physiol. 1994; 480:493–503. PMID: 7869263.
Article16. Johnston GA, Chebib M, Hanrahan JR, Mewett KN. GABAC receptors as drug targets. Curr Drug Targets CNS Neurol Disord. 2003; 2:260–268. PMID: 12871036.17. Choi SE, Choi S, Lee JH, Whiting PJ, Lee SM, Nah SY. Effects of ginsenosides on GABAA receptor channels expressed in Xenopus oocytes. Arch Pharm Res. 2003; 26:28–33. PMID: 12568354.18. Lee BH, Choi SH, Shin TJ, Hwang SH, Kang J, Kim HJ, Kim BJ, Nah SY. Effects of ginsenoside metabolites on GABAA receptor-mediated ion currents. J Ginseng Res. 2012; 36:55–60.19. Goutman JD, Calvo DJ. Studies on the mechanisms of action of picrotoxin, quercetin and pregnanolone at the GABA rho 1 receptor. Br J Pharmacol. 2004; 141:717–727. PMID: 14732759.20. Shimada S, Cutting G, Uhl GR. gamma-Aminobutyric acid A or C receptor? gamma-Aminobutyric acid rho 1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid responses in Xenopus oocytes. Mol Pharmacol. 1992; 41:683–687. PMID: 1314944.21. Johnston GA. GABAC receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol Sci. 1996; 17:319–323. PMID: 8885697.22. Kim JH, Hong YH, Lee JH, Kim DH, Nam G, Jeong SM, Lee BH, Lee SM, Nah SY. A role for the carbohydrate portionof ginsenoside Rg3 in Na+ channelinhibition. Mol Cells. 2005; 19:137–142. PMID: 15750351.23. Lee BH, Jeong SM, Lee JH, Kim DH, Kim JH, Kim JI, Shin HC, Lee SM, Nah SY. Differential effect of ginsenoside metabolites on the 5-HT3A receptor-mediated ion current in Xenopus oocytes. Mol Cells. 2004; 17:51–56. PMID: 15055527.24. Choi SH, Shin TJ, Hwang SH, Lee BH, Kang J, Kim HJ, Jo SH, Choe H, Nah SY. Ginsenoside Rg3 decelerates hERG K+ channel deactivation through Ser631 residue interaction. Eur J Pharmacol. 2011; 663:59–67. PMID: 21586280.25. Feigenspan A, Bormann J. Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J Physiol. 1994; 481:325–330. PMID: 7738828.26. Sine SM, Taylor P. Local anesthetics and histrionicotoxin are allosteric inhibitors of the acetylcholine receptor. Studies of clonal muscle cells. J Biol Chem. 1982; 257:8106–8114. PMID: 7085658.
Article27. Lee BH, Hwang SH, Choi SH, Shin TJ, Kang J, Lee SM, Nah SY. Quercetin inhibits α3β4 nicotinic acetylcholine receptor-mediated ion currents expressed in Xenopus oocytes. Korean J Physiol Pharmacol. 2011; 15:17–22. PMID: 21461236.28. Lee BH, Shin TJ, Hwang SH, Choi SH, Kang J, Kim HJ, Park CW, Lee SH, Nah SY. Inhibitory effects of quercetin on muscle-type of nicotinic acetylcholine receptor-mediated ion currents expressed in Xenopus oocytes. Korean J Physiol Pharmacol. 2011; 15:195–201. PMID: 21994477.29. Shin TJ, Hwang SH, Choi SH, Lee BH, Kang J, Kim HJ, Zukin RS, Rhim H, Nah SY. Effects of protopanaxatriol-ginsenoside metabolites on rat N-methyl-d-aspartic Acid receptor-mediated ion currents. Korean J Physiol Pharmacol. 2012; 16:113–118. PMID: 22563256.30. Wahid F, Jung H, Khan T, Hwang KH, Kim YY. Effects of red ginseng extract on visual sensitivity and ERG b-wave of bullfrog's eye. Planta Med. 2010; 76:426–443. PMID: 19830653.
Article31. Cheung ZH, So KF, Lu Q, Yip HK, Wu W, Shan JJ, Pang PK, Chen CF. Enhanced survival and regeneration of axotomized retinal ganglion cells by a mixture of herbal extracts. J Neurotrauma. 2002; 19:369–378. PMID: 11939504.
Article32. Sen S, Chen S, Wu Y, Feng B, Lui EK, Chakrabarti S. Preventive effects of North American ginseng (Panax quinquefolius) on diabetic retinopathy and cardiomyopathy. Phytother Res. 2013; 27:290–298. PMID: 22566158.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Protopanaxatriol-Ginsenoside Metabolites on Rat N-Methyl-D-Aspartic Acid Receptor-Mediated Ion Currents
- Resveratrol Inhibits GABAC rho Receptor-Mediated Ion Currents Expressed in Xenopus Oocytes
- Effects of Nitric Oxide on Inhibitory Receptors of Rod Bipolar Cells of Rat Retina
- Haloperidol, a typical antipsychotic, inhibits 5-HT3 receptor- mediated currents in NCB-20 cells: a whole-cell patch-clamp study
- Effect of ginsenosides on the desflurane modulation in the recombinant serotonin type 3A receptor expressed in Xenopus laevis oocytes