J Korean Med Sci.
2011 Nov;26(11):1439-1445. 10.3346/jkms.2011.26.11.1439.
Intravenous KITENIN shRNA Injection Suppresses Hepatic Metastasis and Recurrence of Colon Cancer in an Orthotopic Mouse Model
- Affiliations
-
- 1Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea. ijchung@jnu.ac.kr
- 2Department of Surgery, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of Pharmacology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- KMID: 1123443
- DOI: http://doi.org/10.3346/jkms.2011.26.11.1439
Abstract
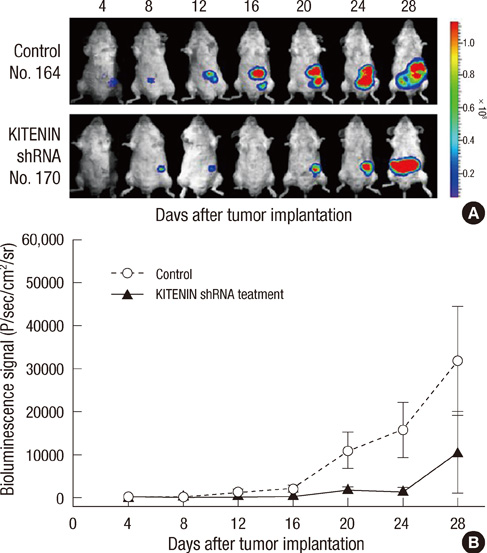
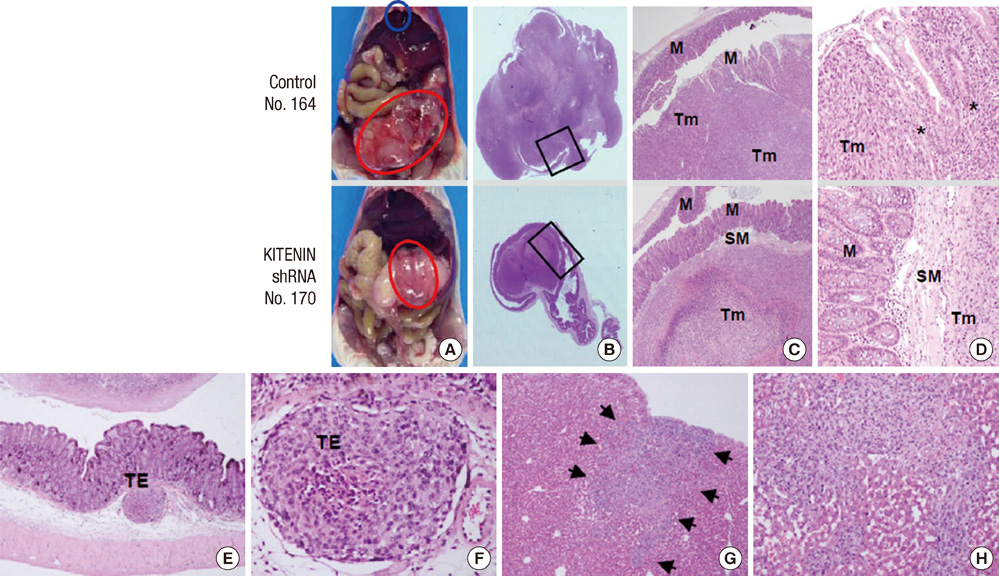
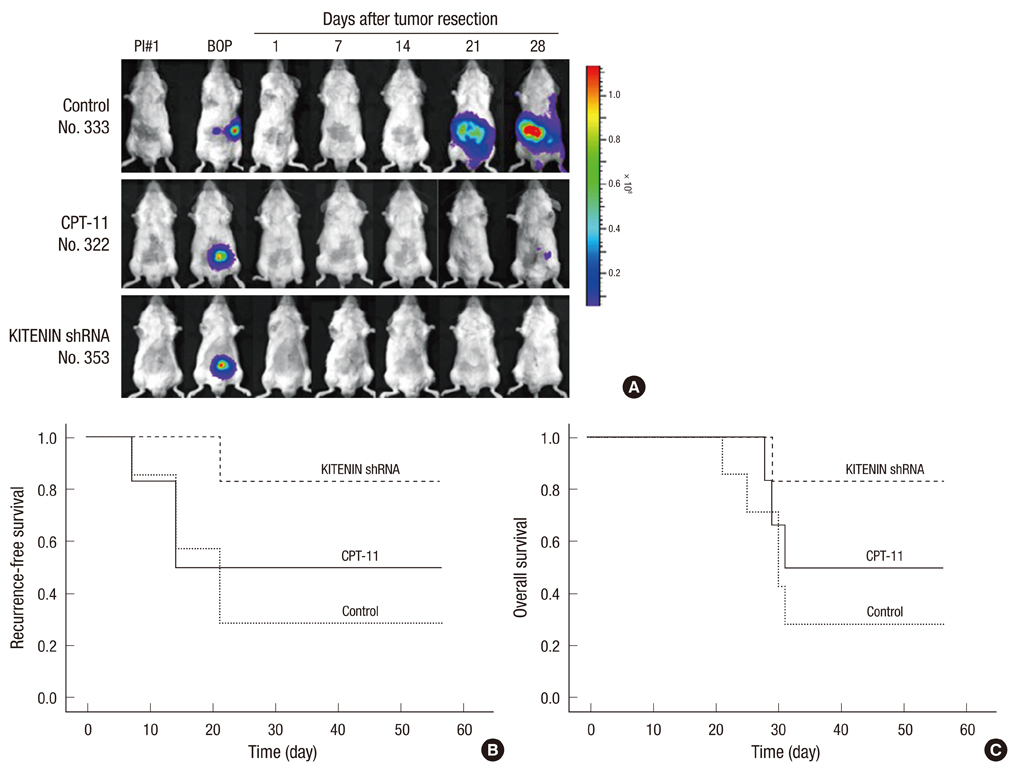
- KITENIN (KAI1 C-terminal interacting tetraspanin) promotes invasion and metastasis in mouse colon cancer models. In the present study, we evaluated the effects of KITENIN knockdown by intravenous administration of short hairpin RNAs (shRNAs) in an orthotopic mouse colon cancer model, simulating a primary or adjuvant treatment setting. We established orthotopic models for colon cancer using BALB/c mice and firefly luciferase-expressing CT-26 (CT26/Fluc) cells. Tumor progression and response to therapy were monitored by bioluminescence imaging (BLI). In the primary therapy model, treatment with KITENIN shRNA substantially delayed tumor growth (P = 0.028) and reduced the incidence of hepatic metastasis (P = 0.046). In the adjuvant therapy model, KITENIN shRNA significantly reduced the extent of tumor recurrence (P = 0.044). Mice treated with KITENIN shRNA showed a better survival tendency than the control mice (P = 0.074). Our results suggest that shRNA targeting KITENIN has the potential to be an effective tool for the treatment of colon cancer in both adjuvant and metastatic setting.
Keyword
MeSH Terms
-
Animals
Carrier Proteins/*genetics/metabolism
Cell Line, Tumor
Colonic Neoplasms/genetics/mortality/pathology/*therapy
Disease Progression
Liver Neoplasms/prevention & control/*secondary
Membrane Proteins/*genetics/metabolism
Mice
Mice, Inbred BALB C
Neoplasm Metastasis/*prevention & control
Neoplasm Recurrence, Local/genetics/*prevention & control
RNA Interference
RNA, Small Interfering/*therapeutic use
Tumor Markers, Biological/genetics
Figure
Reference
-
1. Rinker-Schaeffer CW, Hawkins AL, Ru N, Dong J, Stoica G, Griffin CA, Ichikawa T, Barrett JC, Isaacs JT. Differential suppression of mammary and prostate cancer metastasis by human chromosomes 17 and 11. Cancer Res. 1994. 54:6249–6256.2. Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995. 268:884–886.3. Dong JT, Suzuki H, Pin SS, Bova GS, Schalken JA, Isaacs WB, Barrett JC, Isaacs JT. Down-regulation of the KAI1 metastasis gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res. 1996. 56:4387–4390.4. Adachi M, Taki T, Ieki Y, Huang CI, Higashiyama M, Miyake M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996. 56:1751–1755.5. Schindl M, Birner P, Breitenecker G, Oberhuber G. Downregulation of KAI1 metastasis suppressor protein is associated with a dismal prognosis in epithelial ovarian cancer. Gynecol Oncol. 2001. 83:244–248.6. Su JS, Arima K, Hasegawa M, Franco OE, Umeda Y, Yanagawa M, Sugimura Y, Kawamura J. Decreased expression of KAI1 metastasis suppressor gene is a recurrence predictor in primary pTa and pT1 urothelial bladder carcinoma. Int J Urol. 2004. 11:74–82.7. Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M. Correlation of reduction in MRP/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol. 1998. 153:973–983.8. Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003. 200:39–46.9. Kim JH, Kim MA, Lee HS, Kim WH. Comparative analysis of protein expressions in primary and metastatic gastric carcinomas. Hum Pathol. 2009. 40:314–322.10. Liu WM, Zhang XA. KAI1/CD82, a tumor metastasis suppressor. Cancer Lett. 2006. 240:183–194.11. Jackson P, Marreiros A, Russell PJ. KAI1 tetraspanin and metastasis suppressor. Int J Biochem Cell Biol. 2005. 37:530–534.12. Miranti CK. Controlling cell surface dynamics and signaling: how CD82/KAI1 suppresses metastasis. Cell Signal. 2009. 21:196–211.13. Lee JH, Park SR, Chay KO, Seo YW, Kook H, Ahn KY, Kim YJ, Kim KK. KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Res. 2004. 64:4235–4243.14. Lee JH, Cho ES, Kim MY, Seo YW, Kho DH, Chung IJ, Kook H, Kim NS, Ahn KY, Kim KK. Suppression of progression and metastasis of established colon tumors in mice by intravenous delivery of short interfering RNA targeting KITENIN, a metastasis-enhancing protein. Cancer Res. 2005. 65:8993–9003.15. Kho DH, Bae JA, Lee JH, Cho HJ, Cho SH, Lee JH, Seo YW, Ahn KY, Chung IJ, Kim KK. KITENIN recruits Dishevelled/PKC delta to form a functional complex and controls the migration and invasiveness of colorectal cancer cells. Gut. 2009. 58:509–519.16. Pocard M, Muleris M, Hamelin R, Salmon RJ, Dutrillaux B, Poupon MF. Growth dependency of human colon cancer xenograft on organ environment is related with their original clinical stage. Anticancer Res. 1998. 18:2743–2747.17. Kuo TH, Kubota T, Watanabe M, Furukawa T, Teramoto T, Ishibiki K, Kitajima M, Hoffman RM. Early resection of primary orthotopically-growing human colon tumor in nude mouse prevents liver metastasis: further evidence for patient-like hematogenous metastatic route. Anticancer Res. 1993. 13:293–297.18. Cunningham D, Pyrhönen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998. 352:1413–1418.19. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000. 355:1041–1047.20. Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990. 50:6130–6138.21. Fidler IJ. Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst. 1995. 87:1588–1592.22. Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988. 48:6863–6871.23. Dickson PV, Hamner B, Ng CY, Hall MM, Zhou J, Hargrove PW, McCarville MB, Davidoff AM. In vivo bioluminescence imaging for early detection and monitoring of disease progression in a murine model of neuroblastoma. J Pediatr Surg. 2007. 42:1172–1179.24. Hiraoka K, Kimura T, Logg CR, Tai CK, Haga K, Lawson GW, Kasahara N. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007. 67:5345–5353.25. Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, Ross BD. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia. 2000. 2:491–495.26. Sadikot RT, Blackwell TS. Bioluminescence imaging. Proc Am Thorac Soc. 2005. 2:537–540. 511–512.27. Wang Y, Sun Z, Peng J, Zhan L. Bioluminescent imaging of hepatocellular carcinoma in live mice. Biotechnol Lett. 2007. 29:1665–1670.28. Yanagihara K, Takigahira M, Takeshita F, Komatsu T, Nishio K, Hasegawa F, Ochiya T. A photon counting technique for quantitatively evaluating progression of peritoneal tumor dissemination. Cancer Res. 2006. 66:7532–7539.29. Jones-Bolin S, Zhao H, Hunter K, Klein-Szanto A, Ruggeri B. The effects of the oral, pan-VEGF-R kinase inhibitor CEP-7055 and chemotherapy in orthotopic models of glioblastoma and colon carcinoma in mice. Mol Cancer Ther. 2006. 5:1744–1753.30. Smakman N, Martens A, Kranenburg O, Borel Rinkes IH. Validation of bioluminescence imaging of colorectal liver metastases in the mouse. J Surg Res. 2004. 122:225–230.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene Therapy for Head and Neck Squamous Cell Carcinoma Using KITENIN (KAI1 COOH-Terminal Interacting Tetraspanin)-Antisense Therapy
- Mouse Orthotopic Lung Cancer Model Induced by PC14PE6
- Ultrasonic diagnosis of hepatic metastases in patients with stomach cancer and colon cancer
- TisN0M1 Sigmoid Colon Cancer: A Case Report
- An orthotopic nude mouse model of tongue carcinoma