Yonsei Med J.
2011 May;52(3):463-468. 10.3349/ymj.2011.52.3.463.
Gene Therapy for Head and Neck Squamous Cell Carcinoma Using KITENIN (KAI1 COOH-Terminal Interacting Tetraspanin)-Antisense Therapy
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Chonnam National University Medical School, Hwasun Hospital, Hwasun, Korea. joonkyoo@chonnam.ac.kr
- 2Medical Research Center for Gene Regulation, Chonnam National University Medical School, Hwasun Hospital, Hwasun, Korea.
- 3Department of Nuclear Medicine, Chonnam National University Medical School, Hwasun Hospital, Hwasun, Korea.
- 4Department of Otolaryngology-Head and Neck Surgery, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1777018
- DOI: http://doi.org/10.3349/ymj.2011.52.3.463
Abstract
- PURPOSE
KAI1 COOH-terminal interacting tetraspanin (KITENIN) has been found to act as a promoter of metastasis in murine models of colon cancer and squamous cell carcinoma (SCC). The suppression of tumor progression and metastasis of established colon cancer in mice was observed after intravenous delivery of small interfering RNA (siRNA) targeting KITENIN. The purpose of this study was to investigate the efficacy of gene therapy targeting KITENIN in human head and neck SCC.
MATERIALS AND METHODS
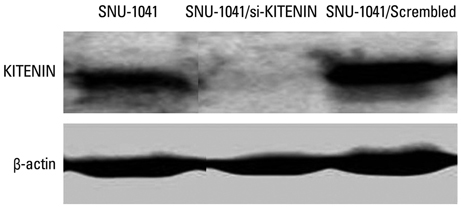
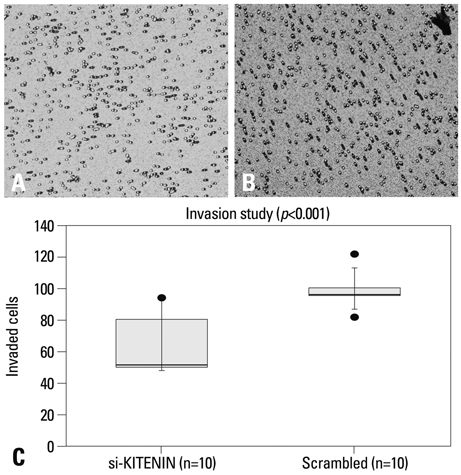
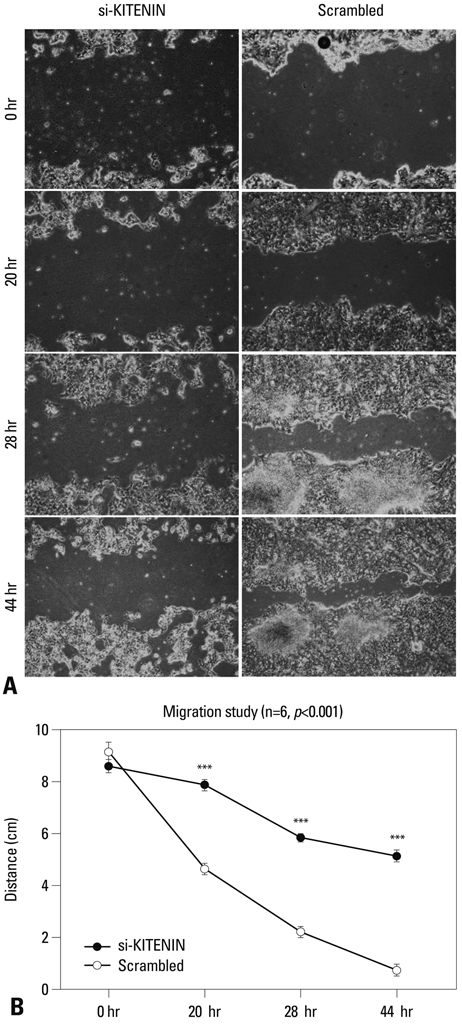
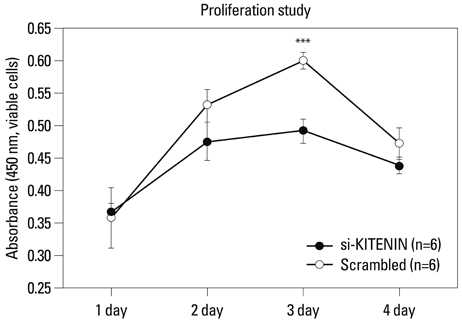
SNU-1041, a well-established human hypopharyngeal SCC cell line, was used. KITENIN expression in SNU-1041 was measured by Western blot analysis. The cells were prepared, maintained in culture dishes with media, and divided into two groups: the si-KITENIN group and the scrambled group (control). The siRNA targeting KITENIN (si-KITENIN) and scrambled DNA were transfected into the SNU-1041 cells in each group. The effect of gene therapy was compared by in vitro experiments to evaluate invasion, migration, and proliferation.
RESULTS
KITENIN was strongly expressed in the SNU-1041 cells, and the number of invaded cells was reduced more in the si-KITENIN group than in the scrambled group (p<0.001). The speed for the narrowing gap, made through adherent cells, was lower in the si-KITENIN group (p<0.001), and the number of viable proliferating cells was reduced in the si-KITENIN group compared to the scrambled group (p<0.001, the third day). KITENIN protein expression was no longer identified in the si-KITENIN group.
CONCLUSION
Gene therapy using an anti-KITENIN strategy might be effective for head and neck squamous carcinoma.
Keyword
MeSH Terms
Figure
Reference
-
1. Howell GM, Grandis JR. Molecular mediators of metastasis in head and neck squamous cell carcinoma. Head Neck. 2005. 27:710–717.
Article2. Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997. 80:1529–1537.
Article3. Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005. 17:559–564.
Article4. Jackson P, Marreiros A, Russell PJ. KAI1 tetraspanin and metastasis suppressor. Int J Biochem Cell Biol. 2005. 37:530–534.
Article5. Takaoka A, Hinoda Y, Satoh S, Adachi Y, Itoh F, Adachi M, et al. Suppression of invasive properties of colon cancer cells by a metastasis suppressor KAI1 gene. Oncogene. 1998. 16:1443–1453.
Article6. Yang JL, Jackson P, Yu Y, Yoshie O, Ham JM, Russell PJ, et al. Invasion and metastasis correlate with down-regulation of KAI1 expression in human colon cancer cell lines. GI Cancer. 2001. 3:313–322.7. Yang X, Wei LL, Tang C, Slack R, Mueller S, Lippman ME. Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res. 2001. 61:5284–5288.8. Jee B, Jin K, Hahn JH, Song HG, Lee H. Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of human prostate cancer cells through Src-dependent pathway. Exp Mol Med. 2003. 35:30–37.
Article9. Lee JH, Seo YW, Park SR, Kim YJ, Kim KK. Expression of a splice variant of KAI1, a tumor metastasis suppressor gene, influences tumor invasion and progression. Cancer Res. 2003. 63:7247–7255.10. Lee JH, Park SR, Chay KO, Seo YW, Kook H, Ahn KY, et al. KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Res. 2004. 64:4235–4243.
Article11. Yagyu R, Hamamoto R, Furukawa Y, Okabe H, Yamamura T, Nakamura Y. Isolation and characterization of a novel human gene, VANGL1, as a therapeutic target for hepatocellular carcinoma. Int J Oncol. 2002. 20:1173–1178.
Article12. Lee JH, Cho ES, Kim MY, Seo YW, Kho DH, Chung IJ, et al. Suppression of progression and metastasis of established colon tumors in mice by intravenous delivery of short interfering RNA targeting KITENIN, a metastasis-enhancing protein. Cancer Res. 2005. 65:8993–9003.
Article13. Lee JK, Bae JA, Sun EG, Kim HD, Yoon TM, Kim K, et al. KITENIN increases invasion and migration of mouse squamous cancer cells and promotes pulmonary metastasis in a mouse squamous tumor model. FEBS Lett. 2009. 583:711–717.
Article14. Lee JK, Lim SC, Kim HD, Yoon TM, Kim K, Nam JH, et al. KITENIN represents a more aggressive phenotype in a murine model of oral cavity squamous carcinoma. Otolaryngol Head Neck Surg. 2010. 142:747–752.
Article15. Lee JK, Yoon TM, Seo DJ, Sun EG, Bae JA, Lim SC, et al. KAI1 COOH-terminal interacting tetraspanin (KITENIN) expression in early and advanced laryngeal cancer. Laryngoscope. 2010. 120:953–958.
Article16. Sioud M. Therapeutic siRNAs. Trends Pharmacol Sci. 2004. 25:22–28.
Article17. Kim SG, Hong JW, Boo SH, Kim MG, Lee KD, Ahn JC, et al. Combination treatment of Cetuximab and photodynamic therapy in SNU-1041 squamous cancer cell line. Oncol Rep. 2009. 22:701–708.
Article18. Koh TY, Park SW, Park KH, Lee SG, Seol JG, Lee DW, et al. Inhibitory effect of p27KIP1 gene transfer on head and neck squamous cell carcinoma cell lines. Head Neck. 2003. 25:44–49.
Article19. Sung MW, Roh JL, Park BJ, Park SW, Kwon TK, Lee SJ, et al. Bile acid induces cyclo-oxygenase-2 expression in cultured human pharyngeal cells: a possible mechanism of carcinogenesis in the upper aerodigestive tract by laryngopharyngeal reflux. Laryngoscope. 2003. 113:1059–1063.
Article20. Martinez LA, Naguibneva I, Lehrmann H, Vervisch A, Tchénio T, Lozano G, et al. Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci U S A. 2002. 99:14849–14854.
Article21. Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002. 2:243–247.
Article22. Scherr M, Battmer K, Winkler T, Heidenreich O, Ganser A, Eder M. Specific inhibition of bcr-abl gene expression by small interfering RNA. Blood. 2003. 101:1566–1569.
Article23. Wilda M, Fuchs U, Wössmann W, Borkhardt A. Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi). Oncogene. 2002. 21:5716–5724.
Article24. Zhang L, Yang N, Mohamed-Hadley A, Rubin SC, Coukos G. Vector-based RNAi, a novel tool for isoform-specific knock-down of VEGF and anti-angiogenesis gene therapy of cancer. Biochem Biophys Res Commun. 2003. 303:1169–1178.
Article25. Holen T, Amarzguioui M, Wiiger MT, Babaie E, Prydz H. Positional effects of short interfering RNAs targeting the human coagulation trigger Tissue Factor. Nucleic Acids Res. 2002. 30:1757–1766.
Article26. Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002. 296:550–553.
Article27. Miyagishi M, Taira K. U6 promoter-driven siRNAs with four uridine 3' overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol. 2002. 20:497–500.
Article28. Lee NS, Dohjima T, Bauer G, Li H, Li MJ, Ehsani A, et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol. 2002. 20:500–505.
Article29. Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002. 20:1006–1010.
Article30. Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003. 33:401–406.
Article31. Shen C, Buck AK, Liu X, Winkler M, Reske SN. Gene silencing by adenovirus-delivered siRNA. FEBS Lett. 2003. 539:111–114.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Herpes Viral Gene Therapy for the Treatment of Head and Neck Squamous Cell Carcinoma
- Intravenous KITENIN shRNA Injection Suppresses Hepatic Metastasis and Recurrence of Colon Cancer in an Orthotopic Mouse Model
- EGFR-targeted Therapy in Head and Neck Squamous Cell Carcinoma
- A Case of Adenosquamous Carcinoma Arising from the Tonsil
- Chemotherapy of Head and Neck Cancer