Korean J Ophthalmol.
2004 Jun;18(1):15-22. 10.3341/kjo.2004.18.1.15.
Ganglion Cell Death in Rat Retinaby Persistent Intraocular Pressure Elevation
- Affiliations
-
- 1Department of Ophthalmology, Kangnam St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1115778
- DOI: http://doi.org/10.3341/kjo.2004.18.1.15
Abstract
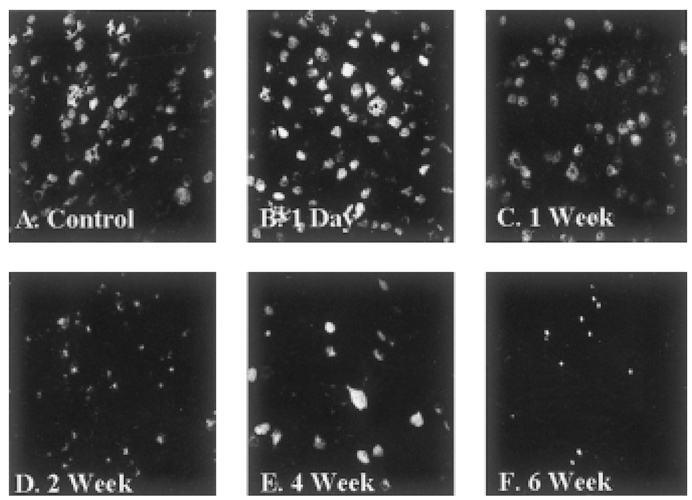
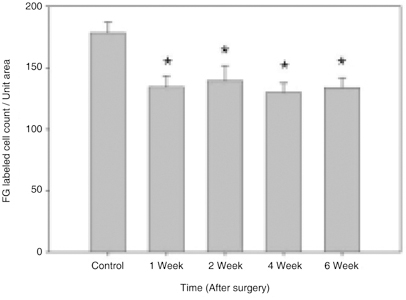
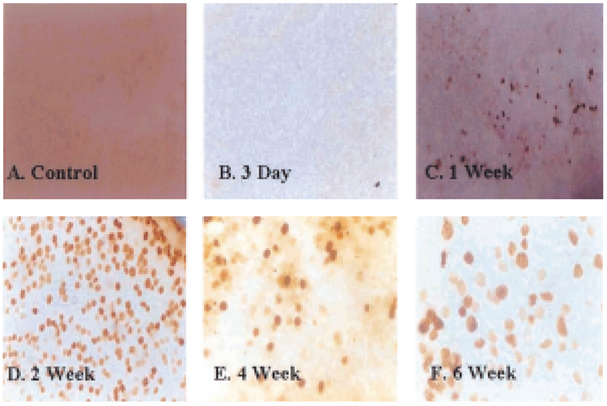
- Glaucoma is characterized by loss of retinal ganglion cells (RGCs) and their axons. Retrograde axoplasmic transport blockade and excitotoxicity were proposed to be a major cause of RGC apoptosis. We conducted this study to characterize the episcleral vessel cauterization glaucoma model in the rat with respect to decreased retrograde axoplasmic flow and subsequent apoptotic RGC death. After episcleral vessels were cauterized in Sprague-Dawley rats, Fluorogold was injected into their superior colliculi by stereotactic method. Retrograde axoplasmic flow and TUNEL-stained apoptotic dead cells were observed microscopically. Elevated intraocular pressure was maintained for up to 6 weeks during follow-up. Retrograde axoplasmic flow to the rat retina was significantly decreased. Apoptotic RGC was selectively TUNELstained in the retina, especially at the ganglion cell layers. We concluded that elevated intraocular pressure caused apoptotic RGC death through retrograde axoplasmic flow blockage. Further studies will elucidate the neuroprotection strategies in glaucoma patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Morrison JC, Fraunfelder FW, Milne ST, Moore CG. Limbal microvasculature of the rat eye. Invest Ophthalmol Vis Sci. 1995. 36:751–756.2. Valenzuela EG, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995. 61:33–44.3. Sawada A, Neufeld AH. Confirmation of the rat model of chronic, moderately elevated intraocular pressure. Exp Eye Res. 1999. 69:525–531.4. Johnson EC, Deppmeier LM, Wentzien SK, Wentzien SK, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000. 41:431–432.5. Jia L, Cepurna WO, Johnson EC, Morrison JC. Patterns of intraocular pressure elevation after aqueous humor outflow obstruction in rats. Invest Ophthalmol Vis Sci. 2000. 41:1380–1385.6. Mittag TW, Danias J, Pohorenec G, Yuan HM, Burakgazi E, Redman RC, Pods SM, Tatton WG. Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2000. 41:3451–3459.7. Bayer AU, Danias J, Brodie S, Maag KP, Chen B, Shen F, Podos SM, Mittag TW. Electroretinographic abnormalities in a rat glaucoma model with chronic elevated intraocular pressure. Exp Eye Res. 2001. 72:667–677.8. Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995. 36:774–786.9. Quigley HA, Mckinnon SJ, Zack DJ, Pease ME, Baumrind LA, Kerrigan FK, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000. 41:3460–3466.10. Ko ML, Hu DN, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000. 41:2967–2971.11. Goldblum D, Mittag T. Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vision Res. 2002. 42:471–478.12. Levkovitch-Verbin H, Quigley HA, Keith R, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci. 2002. 43:402–410.13. Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002. 11:365–370.14. Tatton NA, Tezel Z, Insolia SA, Nandor SA, Edward PD, Wax MB. In situ detection of apoptosis in normal pressure glaucoma: preliminary examination. Surv Ophthalmol. 2001. 45:S268–S272.15. Wang X, Tay SS, Ng YK. An immunohistochemical study of neuronal and glial cell reactions in retinae of rats with experimental glaucoma. Exp Brain Res. 2000. 476–484.16. Wein FB, Levin LA. Current understanding of neuroprotection in glaucoma. Curr Opin Ophthalmol. 2002. 13:61–67.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuronal Cell Death in Reina Ischemia Induced by Elevation of Intraocular Pressure
- Expression of the Na(+)-K(+)-2Cl(-)-Cotransporter 2 in the Normal and Pressure-Induced Ischemic Rat Retina
- Expression of Glutamate Receptors in Experimental Rat Model of Chronic Glaucoma
- Expression of Nitric Oxide Synthase Isoforms in a Rat Model of Chronic Glaucoma
- Intraocular Pressure Changes After Gas Injection into the Rabbit Vitreous Cavity