J Vet Sci.
2006 Sep;7(3):257-262. 10.4142/jvs.2006.7.3.257.
Enhanced immune response with foot and mouth disease virus VP1 and interleukin-1 fusion genes
- Affiliations
-
- 1National Veterinary Research and Quarantine Service, Ministry of Agriculture and Forestry, Anyang 430-824, Korea. parkjh@nvrqs.go.kr
- KMID: 1089910
- DOI: http://doi.org/10.4142/jvs.2006.7.3.257
Abstract
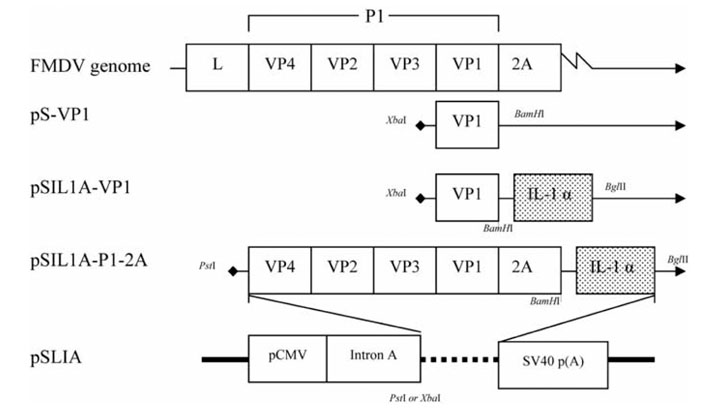
- The capsid of the foot and mouth disease (FMD) virus carries the epitopes that are critical for inducing the immune response. In an attempt to enhance the specific immune response, plasmid DNA was constructed to express VP1/interleukin-1alpha (IL-1alpha) and precursor capsid (P1) in combination with 2A (P1-2A)/IL-1alpha under the control of the human cytomegalovirus (HCMV) immediateearly promoter and intron. After DNA transfection into MA104 (monkey kidney) cells, Western blotting and an immunofluorescence assay were used to confirm the expression of VP1 or P1-2A and IL-1alpha. Mice were inoculated with the encoding plasmids via the intradermal route, and the IgG1 and IgG2a levels were used to determine the immune responses. These results show that although the immunized groups did not carry a high level of neutralizing antibodies, the plasmids encoding the VP1/ IL-1alpha, and P1-2A /IL-1alpha fused genes were effective in inducing an enhanced immune response.
MeSH Terms
-
Animals
Antibodies, Viral/blood
Capsid Proteins/biosynthesis/genetics/*immunology
Cell Line
DNA, Viral/genetics
Enzyme-Linked Immunosorbent Assay
Foot-and-Mouth Disease/*immunology/prevention&control
Foot-and-Mouth Disease Virus/genetics/*immunology
Haplorhini
Immunization
Interleukin-1/biosynthesis/genetics/*immunology
Male
Mice
Mice, Inbred C57BL
Plasmids/genetics
Polymerase Chain Reaction
Recombinant Fusion Proteins/biosynthesis/genetics/immunology
Specific Pathogen-Free Organisms
Transfection
Vaccines, DNA/genetics/*immunology
Figure
Cited by 2 articles
-
Recombinant DNA and Protein Vaccines for Foot-and-mouth Disease Induce Humoral and Cellular Immune Responses in Mice
Ji-young Bae, Sun-Hwa Moon, Jung-Ah Choi, Jong-Sug Park, Bum-Soo Hahn, Ki-Yong Kim, Byunghan Kim, Jae-Young Song, Dae-Hyuck Kwon, Suk-Chan Lee, Jong-Bum Kim, Joo-Sung Yang
Immune Netw. 2009;9(6):265-273. doi: 10.4110/in.2009.9.6.265.Strategy for Novel Vaccine and Antivirals Against Foot-and-Mouth Disease
Jong-Hyeon Park, Su-Mi Kim, Kwang-Nyeong Lee, Young-Joon Ko, Hyang-Sim Lee, In-Soo Cho
J Bacteriol Virol. 2010;40(1):1-10. doi: 10.4167/jbv.2010.40.1.1.
Reference
-
1. Abu Elzein EM, Crowther JR. Enzyme-labelled immunosorbent assay techniques in foot-and-mouth disease virus research. J Hyg (Lond). 1978. 80:391–399.
Article2. Bachrach HL. Foot-and-mouth disease. Annu Rev Microbiol. 1968. 22:201–244.
Article3. Douvdevani A, Huleihel M, Zoller M, Segal S, Apte RN. Reduced tumorigenicity of fibrosarcomas which constitutively generate IL-1 alpha either spontaneously or following IL-1 alpha gene transfer. Int J Cancer. 1992. 51:822–830.
Article4. Edward S. Manual of Diagnostic Tests and Vaccines for Terrestrial. 2004. 5th ed. Paris: OIE;111–128.5. Elias JA, Lentz V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL-6 production and stabilize IL-6 messenger RNA. J Immunol. 1990. 145:161–166.6. Flint SJ, Enquist LW, Krug RM, Racaniello VR, Skalka AM. Principles of Virology. 2000. Washington DC: ASM Press;478–516.7. Giese M. DNA-antiviral vaccines: new developments and approaches-a review. Virus Genes. 1998. 17:219–232.8. Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999. 18:765–777.
Article9. Nobiron I, Thompson I, Brownlie J, Collins ME. Cytokine adjuvancy of BVDV DNA vaccine enhances both humoral and cellular immune responses in mice. Vaccine. 2001. 19:4226–4235.
Article10. Rodriguez A, Ley V, Ortuno E, Ezquerra A, Saalmuller A, Sobrino F, Saiz JC. A porcine CD8+ T cell clone with heterotypic specificity for foot-and-mouth disease virus. J Gen Virol. 1996. 77:2089–2096.
Article11. Shaw AR. Thomson AW, editor. Molecular Biology of Cytokines. The Cytokine Handbook. 1991. San Diego: Academic Press;19–46.12. Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, Hawe LA, Leander KR, Martinez D, Perry HC, Shiver JW, Montgomery DL, Liu MA. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993. 259:1745–1749.
Article13. Wong HT, Cheng SC, Sin FW, Chan EW, Sheng ZT, Xie Y. A DNA vaccine against foot-and-mouth disease elicits an immune response in swine which is enhanced by co-administration with interleukin-2. Vaccine. 2002. 20:2641–2647.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeted Delivery of VP1 Antigen of Foot-and-mouth Disease Virus to M Cells Enhances the Antigen-specific Systemic and Mucosal Immune Response
- Novel pan-lineage VP1 specific degenerate primers for precise genetic characterization of serotype O foot and mouth disease virus circulating in India
- Recombinant DNA and Protein Vaccines for Foot-and-mouth Disease Induce Humoral and Cellular Immune Responses in Mice
- Molecular characterization of a 13-amino acid deletion in VP1 (1D) protein and novel amino acid substitutions in 3D polymerase protein of foot and mouth disease virus subtype A/Iran87
- Effect of simultaneous administration of foot-and-mouth disease (FMD) and anthrax vaccines on antibody response to FMD in sheep