J Vet Sci.
2011 Dec;12(4):363-371. 10.4142/jvs.2011.12.4.363.
Molecular characterization of a 13-amino acid deletion in VP1 (1D) protein and novel amino acid substitutions in 3D polymerase protein of foot and mouth disease virus subtype A/Iran87
- Affiliations
-
- 1Department of Biotechnology, Razi Vaccine and Serum Research Institute, Karaj 3197619751, Iran. m.esmaelizad@rvsri.ir
- 2Department of Quality Control, Razi Vaccine and Serum Research Institute, Karaj 3197619751, Iran.
- KMID: 1365020
- DOI: http://doi.org/10.4142/jvs.2011.12.4.363
Abstract
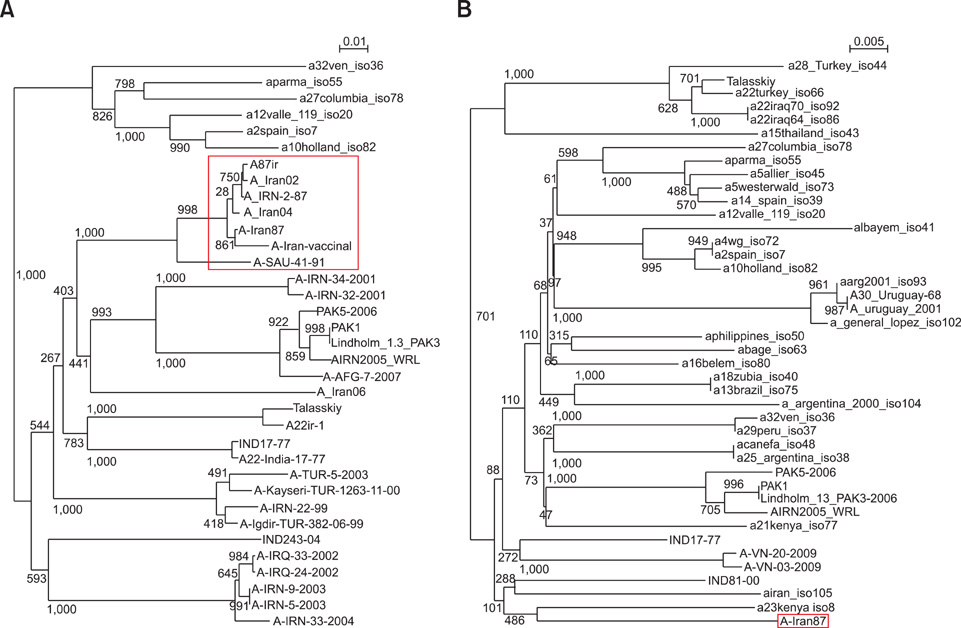
- The nucleotide sequence of the VP1 (1D) and partial 3D polymerase (3Dpol) coding regions of the foot and mouth disease virus (FMDV) vaccine strain A/Iran87, a highly passaged isolate (~150 passages), was determined and aligned with previously published FMDV serotype A sequences. Overall analysis of the amino acid substitutions revealed that the partial 3Dpol coding region contained four amino acid alterations. Amino acid sequence comparison of the VP1 coding region of the field isolates revealed deletions in the highly passaged Iranian isolate (A/Iran87). The prominent G-H loop of the FMDV VP1 protein contains the conserved arginine-glycine-aspartic acid (RGD) tripeptide, which is a well-known ligand for a specific cell surface integrin. Despite losing the RGD sequence of the VP1 protein and an Asp26-->Glu substitution in a beta sheet located within a small groove of the 3Dpol protein, the virus grew in BHK 21 suspension cell cultures. Since this strain has been used as a vaccine strain, it may be inferred that the RGD deletion has no critical role in virus attachment to the cell during the initiation of infection. It is probable that this FMDV subtype can utilize other pathways for cell attachment.
Keyword
MeSH Terms
-
Amino Acid Sequence
Amino Acid Substitution
Antigens, Viral/chemistry/*genetics/metabolism
Capsid Proteins/chemistry/*genetics/metabolism
Cloning, Molecular
Foot-and-Mouth Disease Virus/classification/*genetics/*metabolism
Gene Expression Regulation, Viral
Molecular Sequence Data
Phylogeny
Viral Nonstructural Proteins/chemistry/*genetics/metabolism
Figure
Reference
-
1. Araújo JP Jr, Montassier HJ, Pinto AA. Extensive antigenic and genetic variation among foot-and-mouth disease type A viruses isolated from the 1994 and 1995 foci in São Paulo, Brazil. Vet Microbiol. 2002. 84:15–27.
Article2. Biswas S, Sanyal A, Hemadri D, Tosh C, Mohapatra JK, Manoj Kumar R, Bandyopadhyay SK. Sequence analysis of the non-structural 3A and 3C protein-coding regions of foot-and-mouth disease virus serotype Asia1 field isolates from an endemic country. Vet Microbiol. 2006. 116:187–193.
Article3. Chen W, Yan W, Du Q, Fei L, Liu M, Ni Z, Sheng Z, Zheng Z. RNA interference targeting VP1 inhibits foot-and-mouth disease virus replication in BHK-21 cells and suckling mice. J Virol. 2004. 78:6900–6907.
Article4. Chen X, Feng Q, Wu Z, Liu Y, Huang K, Shi R, Chen S, Lu W, Ding M, Collins RA, Fung YW, Lau LT, Yu AC, Chen J. RNA-dependent RNA polymerase gene sequence from foot-and-mouth disease virus in Hong Kong. Biochem Biophys Res Commun. 2003. 308:899–905.
Article5. Du J, Chang H, Cong G, Shao J, Lin T, Shang Y, Liu Z, Liu X, Cai X, Xie Q. Complete nucleotide sequence of a Chinese serotype Asia1 vaccine strain of foot-and-mouth disease virus. Virus Genes. 2007. 35:635–642.
Article6. Feng Q, Chen X, Ma O, Liu Y, Ding M, Collins RA, Ko LS, Xing J, Lau LT, Yu AC, Chen J. Serotype and VP1 gene sequence of a foot-and-mouth disease virus from Hong Kong (2002). Biochem Biophys Res Commun. 2003. 302:715–721.
Article7. Ferrer-Orta C, Agudo R, Domingo E, Verdaguer N. Structural insights into replication initiation and elongation processes by the FMDV RNA-dependent RNA polymerase. Curr Opin Struct Biol. 2009. 19:752–758.
Article8. Frimann TH, Barfoed AM, Aasted B, Kamstrup S. Vaccination of mice with plasmids expressing processed capsid protein of foot-and-mouth disease virus-Importance of dominant and subdominant epitopes for antigenicity and protection. Vaccine. 2007. 25:6191–6200.
Article9. Gerner W, Denyer MS, Takamatsu HH, Wileman TE, Wiesmüller KH, Pfaff E, Saalmüller A. Identification of novel foot-and-mouth disease virus specific T-cell epitopes in c/c and d/d haplotype miniature swine. Virus Res. 2006. 121:223–228.
Article10. Jangra RK, Tosh C, Sanyal A, Hemadri D, Bandyopadhyay SK. Antigenic and genetic analyses of foot-and-mouth disease virus type A isolates for selection of candidate vaccine strain reveals emergence of a variant virus that is responsible for most recent outbreaks in India. Virus Res. 2005. 112:52–59.
Article11. Jaulent AM, Fahy AS, Knox SR, Birtley JR, Roqué-Rosell N, Curry S, Leatherbarrow RJ. A continuous assay for foot-and-mouth disease virus 3C protease activity. Anal Biochem. 2007. 368:130–137.
Article12. Malirat V, de Barros JJF, Bergmann IE, Campo RM, Neitzert E, da Costa EV, da Silva EE, Falczuk AJ, Pinheiro DSB, de Vergara N, Cirvera JLQ, Maradei E, Di Landro R. Phylogenetic analysis of foot-and-mouth disease virus type O re-emerging in free areas of South America. Virus Res. 2007. 124:22–28.
Article13. Marquardt O, Freiberg B. Antigenic variation among foot-and-mouth disease virus type A field isolates of 1997-1999 from Iran. Vet Microbiol. 2000. 74:377–386.
Article14. Mason PW, Grubman MJ, Baxt B. Molecular basis of pathogenesis of FMDV. Virus Res. 2003. 91:9–32.
Article15. McKenna TS, Lubroth J, Rieder E, Baxt B, Mason PW. Receptor binding site-deleted foot-and-mouth disease (FMD) virus protects cattle from FMD. J Virol. 1995. 69:5787–5790.
Article16. Peng JM, Liang SM, Liang CM. VP1 of foot-and-mouth disease virus induces apoptosis via the Akt signaling pathway. J Biol Chem. 2004. 279:52168–52174.
Article17. Perez AM, Thurmond MC, Grant PW, Carpenter TE. Use of the scan statistic on disaggregated province-based data: foot-and-mouth disease in Iran. Prev Vet Med. 2005. 71:197–207.
Article18. Storey P, Theron J, Maree FF, O'Neill HG. A second RGD motif in the 1D capsid protein of a SAT1 type foot-and-mouth disease virus field isolate is not essential for attachment to target cells. Virus Res. 2007. 124:184–192.
Article19. Tosh C, Hemadri D, Sanyal A. Evidence of recombination in the capsid-coding region of type A foot-and-mouth disease virus. J Gen Virol. 2002. 83:2455–2460.
Article20. Wang JL, Liu MQ, Han J, Chen WZ, Cong W, Cheng G, Gao YH, Lu YG, Chen JL, Zuo XP, Yan WY, Zheng ZX. A peptide of foot-and-mouth disease virus serotype Asia1 generating a neutralizing antibody response, and an immunostimulatory peptide. Vet Microbiol. 2007. 125:224–231.
Article21. Weddell GN, Yansura DG, Dowbenko DJ, Hoathn ME, Grubman MJ, Moore DM, Kleid DG. Sequence variation in the gene for the immunogenic capsid proteinVP1 of foot-and-mouth disease virus type A. Proc Natl Acad Sci USA. 1985. 82:2618–2622.
Article22. Yang M, Clavijo A, Li M, Hole K, Holland H, Wang H, Deng MY. Identification of a major antibody binding epitope in the non-structural protein 3D of foot-and-mouth disease virus in cattle and the development of a monoclonal antibody with diagnostic applications. J Immunol Methods. 2007. 321:174–181.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sequence and phylogenetic analysis of the non-structural 3A and 3B protein-coding regions of foot-and-mouth disease virus subtype A Iran 05
- Identification and antigenic site analysis of foot-and-mouth disease virus from pigs and cattle in Korea
- Amino Acid Transporters as Potential Therapeutic Targets in Thyroid Cancer
- Novel foot-and-mouth disease virus in Korea, July-August 2014
- Molecular characterization and genogrouping of VP1 of aquatic birnavirus GC1 isolated from rockfish Sebastes schlegeli in Korea