J Vet Sci.
2006 Dec;7(4):309-314. 10.4142/jvs.2006.7.4.309.
Gamma-ray irradiation stimulates the expression of caveolin-1 and GFAP in rat spinal cord: a study of immunoblot and immunohistochemistry
- Affiliations
-
- 1Department of Veterinary Medicine, College of Applied Life Sciences, Cheju National University, Jeju 690-756, Korea. shint@cheju.ac.kr
- 2Department of Biochemistry, College of Medicine, Cheju National University, Jeju 690-756, Korea.
- 3Department of Nuclear and Energy Engineering, College of Engineering, Cheju National University, Jeju 690-756, Korea.
- 4Applied Radiological Science Research Institute, Cheju National University, Jeju 690-756, Korea.
- KMID: 1089475
- DOI: http://doi.org/10.4142/jvs.2006.7.4.309
Abstract
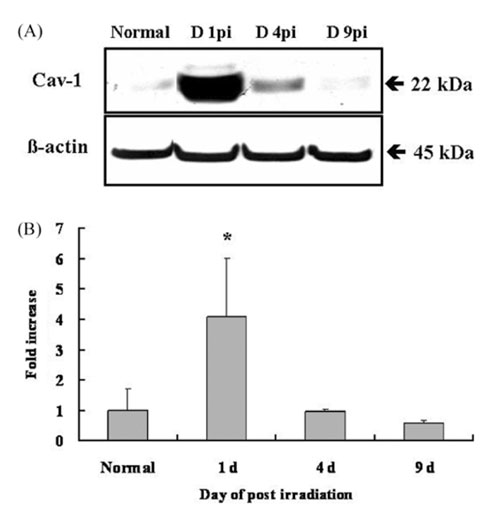
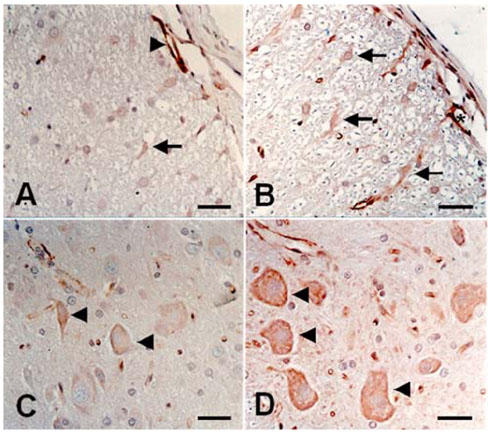
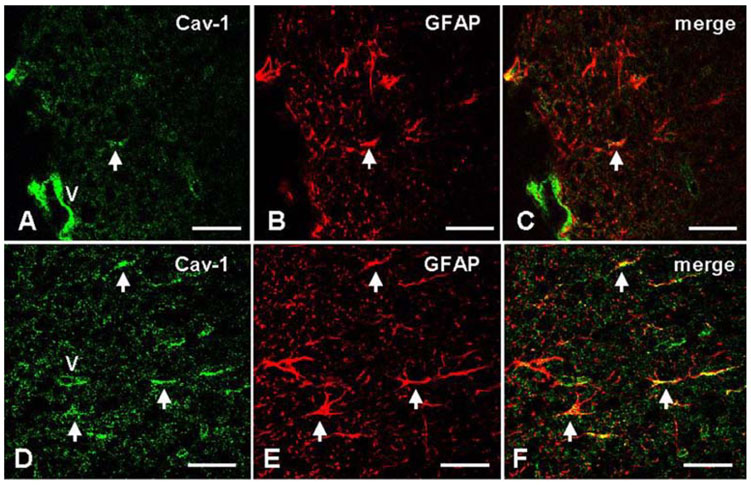
- We studied the expression of caveolin-1 in the spinal cords of rats using 60Co gamma-ray irradiation (single dose of 8 Gray (Gy)) in order to determine the possible involvement of caveolin-1 in the tissues of the central nervous system after irradiation. Spinal cords sampled at days 1, 4, and 9 post-irradiation (PI) (n = 5 per each time point) were analyzed by Western blot and immunohistochemistry. Western blot analysis showed that the expression of caveolin-1 was significantly increased at day 1 PI (p < 0.05), and returned to the level of normal control rats on days 4 and 9 PI. Immunohistochemistry showed that caveolin-1 immunoreactivity was enhanced in some glial cells, vascular endothelial cells, and neurons in the spinal cords. The increased expression of glial fibrillary acidic protein (GFAP), a marker for an astroglial reaction, was consistent with that of caveolin-1. In addition, caveolin-1 was co-localized in hypertrophied GFAP-positive astrocytes. Taking all these facts into consideration, we postulate that irradiation induces the increased expression of caveolin-1 in cells of the central nervous system, and that its increased expression in astrocytes may contribute to hypertrophy of astrocytes in the spinal cord after irradiation. The precise role of caveolin-1 in the spinal cords should be studied further.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The radioprotective effects of the hexane and ethyl acetate extracts of Callophyllis japonica in mice that undergo whole body irradiation
Jeongtae Kim, Changjong Moon, Heechul Kim, Jinwoo Jeong, Juyeon Lee, Jihoon Kim, Jin Won Hyun, Jae Woo Park, Mi Yeon Moon, Nam Ho Lee, Sung Ho Kim, Youngheun Jee, Taekyun Shin
J Vet Sci. 2008;9(3):281-284. doi: 10.4142/jvs.2008.9.3.281.
Reference
-
1. Atkinson SL, Li YQ, Wong CS. Apoptosis and proliferation of oligodendrocyte progenitor cells in the irradiated rodent spinal cord. Int J Radiat Oncol Biol Phys. 2005. 62:535–544.
Article2. Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer. 1985. 56:1106–1111.
Article3. Bushby KM. The limb-girdle muscular dystrophies-multiple genes, multiple mechanisms. Hum Mol Genet. 1999. 8:1875–1882.4. Cameron PL, Liu C, Smart DK, Hantus ST, Fick JR, Cameron RS. Caveolin-1 expression is maintained in rat and human astroglioma cell lines. Glia. 2002. 37:275–290.
Article5. Colasanti M, Persichini T, Fabrizi C, Cavalieri E, Venturini G, Ascenzi P, Lauro GM, Suzuki H. Expression of a NOS-III-like protein in human astroglial cell culture. Biochem Biophys Res Commun. 1998. 252:552–555.
Article6. Fisher BJ, Scott C, Macdonald DR, Coughlin C, Curran WJ. Phase I study of topotecan plus cranial radiation for glioblastoma multiforme: results of Radiation Therapy Oncology Group Trial 9507. J Clin Oncol. 2001. 19:1111–1117.
Article7. Hassan GS, Williams TM, Frank PG, Lisanti MP. Caveolin-1-deficient aortic smooth muscle cells show cell autonomous abnormalities in proliferation, migration, and endothelin-based signal transduction. Am J Physiol Heart Circ Physiol. 2006. 290:H2393–H2401.
Article8. Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH, Han IO. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 2006. 21:457–467.
Article9. Ikezu T, Ueda H, Trapp BD, Nishiyama K, Sha JF, Volonte D, Galbiati F, Byrd AL, Bassell G, Serizawa H, Lane WS, Lisanti MP, Okamoto T. Affinity-purification and characterization of caveolins from the brain: differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 1998. 804:177–192.
Article10. Janeczko K, Pawlinski R, Setkowicz Z, Ziaja M, Soltys Z, Ryszka A. Long-term postnatal effect of prenatal irradiation on the astrocyte proliferative response to brain injury. Brain Res. 1997. 770:237–241.
Article11. Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003. 63:5950–5956.12. Moore AH, Olschowka JA, Williams JP, Paige SL, O'Banion MK. Radiation-induced edema is dependent on cyclooxygenase 2 activity in mouse brain. Radiat Res. 2004. 161:153–160.
Article13. Nordal RA, Wong CS. Intercellular adhesion molecule-1 and blood-spinal cord barrier disruption in central nervous system radiation injury. J Neuropathol Exp Neurol. 2004. 63:474–483.
Article14. Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J Biol Chem. 1998. 273:5419–5422.
Article15. Podar K, Anderson KC. Caveolin-1 as a potential new therapeutic target in multiple myeloma. Cancer Lett. 2006. 233:10–15.
Article16. Shin T, Kim H, Jin JK, Moon C, Ahn M, Tanuma N, Matsumoto Y. Expression of caveolin-1, -2, and -3 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005. 165:11–20.
Article17. Silva WI, Maldonado HM, Velazquez G, Rubio-Davila M, Miranda JD, Aquino E, Mayol N, Cruz-Torres A, Jardon J, Salgado-Villanueva IK. Caveolin isoform expression during differentiation of C6 glioma cells. Int J Dev Neurosci. 2005. 23:599–612.
Article18. Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000. 153:357–370.
Article19. Williams TM, Hassan GS, Li J, Cohen AW, Medina F, Frank PG, Pestell RG, Di Vizio D, Loda M, Lisanti MP. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice. J Biol Chem. 2005. 280:25134–25145.
Article20. Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004. 36:584–595.
Article21. Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005. 288:C494–C506.
Article22. Yang T, Wu SL, Liang JC, Rao ZR, Ju G. Time-dependent astroglial changes after gamma knife radiosurgery in the rat forebrain. Neurosurgery. 2000. 47:407–415.
Article23. Zhang SX, Geddes JW, Owens JL, Holmberg EG. X-irradiation reduces lesion scarring at the contusion site of adult rat spinal cord. Histol Histopathol. 2005. 20:519–530.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased phosphorylation of caveolin-1 in the spinal cord of irradiated rats
- Ectopic Expression of Caveolin-1 Induces COX-2 Expression in Rabbit Articular Chondrocytes via MAP Kinase Pathway
- Immunohistochemical study of caveolin-1 and -2 in the rat retina
- Expression of Caveolin-3 in the Myelin Sheath of Peripheral Nerve
- The Effects of Nimodipine on Irradiation-Induced Apoptosis in the Rat Spinal Cord