J Vet Sci.
2006 Jun;7(2):101-104. 10.4142/jvs.2006.7.2.101.
Immunohistochemical study of caveolin-1 and -2 in the rat retina
- Affiliations
-
- 1Department of Veterinary Medicine, Cheju National University, Jeju 690-756, Korea. shint@cheju.ac.kr
- 2Department of Veterinary Medicine, Kangwon National University, Chunchon 200-701, Korea.
- KMID: 1059192
- DOI: http://doi.org/10.4142/jvs.2006.7.2.101
Abstract
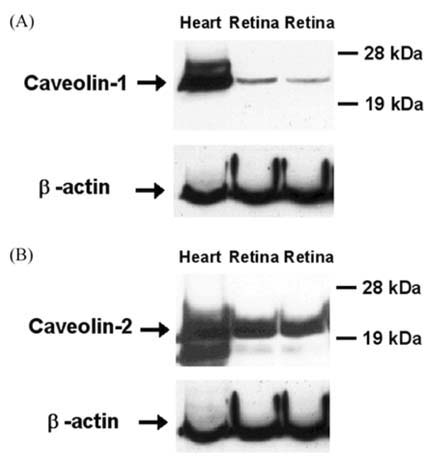
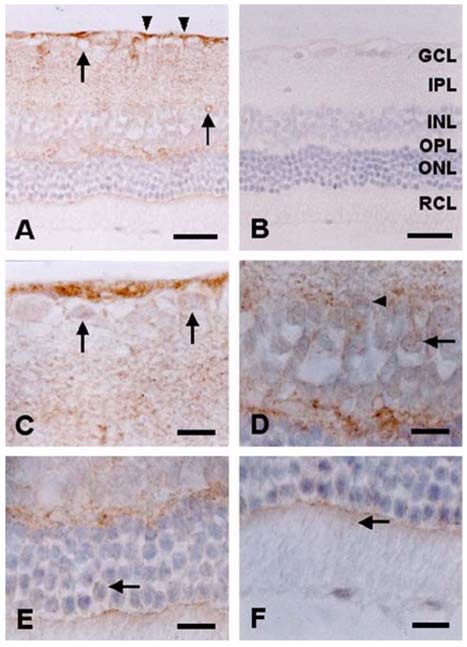
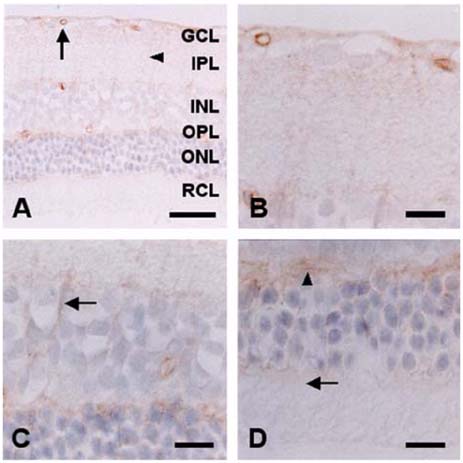
- The expression of caveolin-1 and -2 in the retina was examined; Western blot analysis showed that both were present. Immunohistochemistry indicated that caveolin-1 was expressed in the majority of retinal layers, including the ganglion cell layer, inner plexiform layer, outer plexiform layer, and in the vascular endothelial cells of the retina. Caveolin-2 was primarily immunostained in the vessels, but in a few other elements as well. This is the first demonstration of caveolin differential expression in the retina of rats, and suggests that caveolin plays an important role in signal transduction in glial cells and neuronal cells.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Distribution of caveolin isoforms in the lemur retina
Ágnes I Berta, Anna L Kiss, Ákos Lukáts, Arnold Szabó, Ágoston Szél
J Vet Sci. 2007;8(3):295-297. doi: 10.4142/jvs.2007.8.3.295.
Reference
-
1. Bridges CC, El-Sherbeny A, Roon P, Ola MS, Kekuda R, Ganapathy V, Camero RS, Cameron PL, Smith SB. A comparison of caveolae and caveolin-1 to folate receptor alpha in retina and retinal pigment epithelium. Histochem J. 2001. 33:149–158.2. Elliott MH, Fliesler SJ, Ghalayini AJ. Cholesterol-dependent association of caveolin-1 with the transducin alpha subunit in bovine photoreceptor rod outer segments: disruption by cyclodextrin and guanosine 5'-O-(3-thiotriphosphate). Biochemistry. 2003. 42:7892–7903.
Article3. Epand RM, Sayer BG, Epand RF. Caveolin scaffolding region and cholesterol-rich domains in membranes. J Mol Biol. 2005. 345:339–350.
Article4. Feng Y, Venema VJ, Venema RC, Tsai N, Caldwell RB. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem Biophys Res Commun. 1999. 256:192–197.
Article5. Gaudreault SB, Blain JF, Gratton JP, Poirier J. A role for caveolin-1 in post-injury reactive neuronal plasticity. J Neurochem. 2005. 92:831–839.
Article6. Ikezu T, Ueda H, Trapp BD, Nishiyama K, Sha JF, Volonte D, Galbiati F, Byrd AL, Bassell G, Serizawa H, Lane WS, Lisanti MP, Okamoto T. Affinity-purification and characterization of caveolins from the brain: differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 1998. 804:177–192.
Article7. Kachi S, Yamazaki A, Usukura J. Localization of caveolin-1 in photoreceptor synaptic ribbons. Invest Ophthalmol Vis Sci. 2001. 42:850–852.8. Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett. 2004. 9:195–220.9. Russelakis-Carneiro M, Hetz C, Maundrell K, Soto C. Prion replication alters the distribution of synaptophysin and caveolin 1 in neuronal lipid rafts. Am J Pathol. 2004. 165:1839–1848.
Article10. Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997. 272:29337–29346.11. Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996. 93:131–135.
Article12. Shin T, Kim H, Jin JK, Moon C, Ahn M, Tanuma N, Matsumoto Y. Expression of caveolin-1, -2, and -3 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005. 165:11–20.
Article13. Shin T, Kim M. Effects of kainic acid on the duck retina. Korean J Vet Res. 1996. 26:221–224.14. Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996. 271:15160–15165.15. Turi A, Kiss AL, Mullner N. Estrogen downregulates the number of caveolae and the level of caveolin in uterine smooth muscle. Cell Biol Int. 2001. 25:785–794.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution of caveolin isoforms in the lemur retina
- Expression of Caveolin-3 in the Myelin Sheath of Peripheral Nerve
- Immunohistochemical localization of heme oxygenase isozymes in the aged rat retina
- Decreased urothelial expression of caveolin 1 and 2 in aging rats showing detrusor overactivity: Potential association with aging bladder
- Expression of Caveolin-3 in the Muscle Cell and Tissue