Korean J Radiol.
2012 Feb;13(1):82-89. 10.3348/kjr.2012.13.1.82.
Diffusion-Weighted Imaging of a Prostate Cancer Xenograft Model Seen on a 7 Tesla Animal MR Scanner: Comparison of ADC Values and Pathologic Findings
- Affiliations
-
- 1Department of Radiology, Yonsei University College of Medicine, Severance Hospital, Seoul 120-752, Korea.
- 2Department of Radiology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Gyeonggi-do 463-707, Korea. hakjlee@radiol.snu.ac.kr
- 3Department of Pathology, Hallym University Sacred Heart Hospital, Gyeonggi-do 431-070, Korea.
- 4Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Gyeonggi-do 463-707, Korea.
- 5Genitourinary Cancer Branch, Research Institute and Hospital, National Cancer Center, Gyeonggi-do 410-769, Korea.
- 6Department of Radiology, Research Institute and Hospital, National Cancer Center, Gyeonggi-do 410-769, Korea.
- 7Department of Molecular Imaging & Therapy Branch, Research Institute and Hospital, National Cancer Center, Gyeonggi-do 410-769, Korea.
- KMID: 1058797
- DOI: http://doi.org/10.3348/kjr.2012.13.1.82
Abstract
OBJECTIVE
To assess the relationship between apparent diffusion coefficient (ADC) values on diffusion-weighted magnetic resonance (MR) imaging and pathologic measures of a tumor using a prostate cancer xenograft model.
MATERIALS AND METHODS
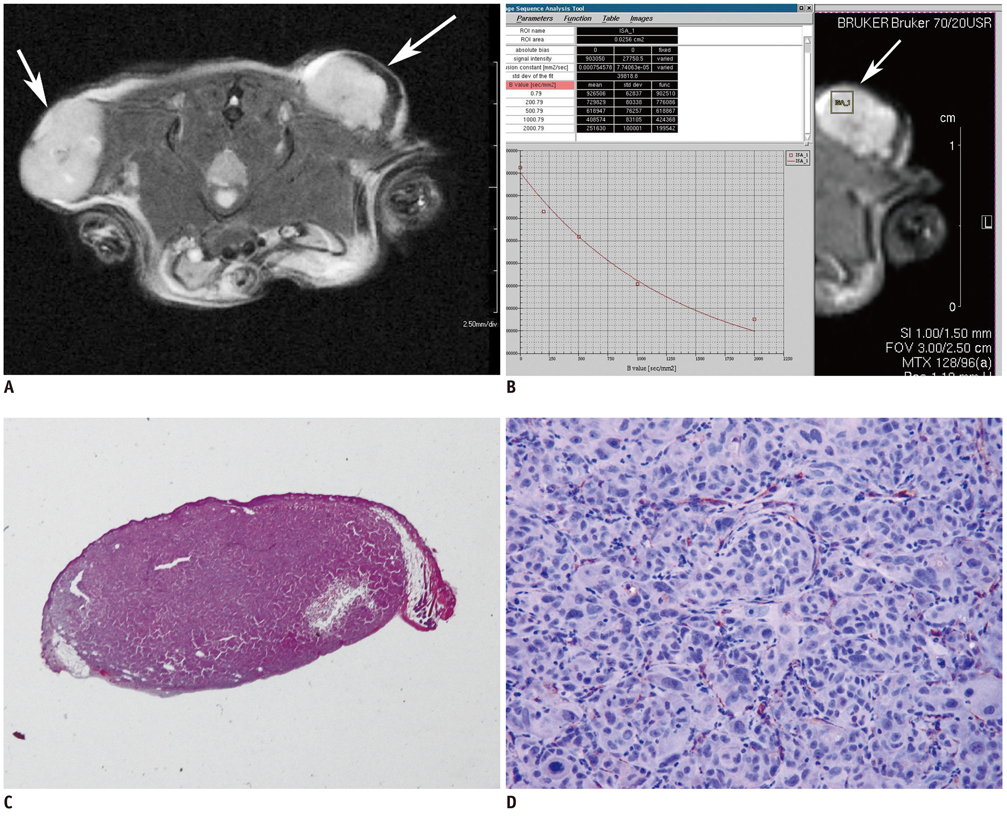
Eighteen athymic nude mice with 36 PC-3-induced tumors were sacrificed to obtain specimens immediately after MR imaging in order to compare the findings on MR images with those seen on pathological specimens. Using a high-field small-animal MR scanner, T1- and T2-weighted imaging and DW MR imaging was performed. Tumors were then processed for Hematoxylin and Eosin staining to evaluate tumor cellularity, intratumoral necrosis and immunostaining using antibodies directed against CD31 and vascular endothelial growth factor (VEGF) to determine the levels of microvessel density (MVD). Mean ADC values that were measured on the solid portion within each tumor were compared with tumor volume, cellularity, degree of necrosis, VEGF expression, and MVD in the corresponding section of the pathological specimen.
RESULTS
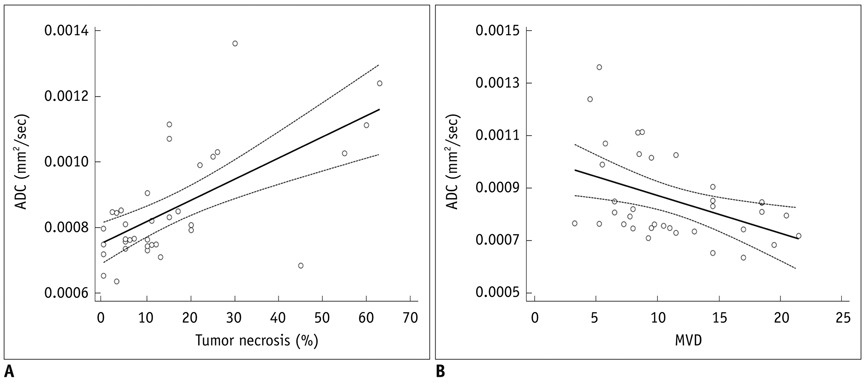
Mean ADC values of the solid portion within the PC-3-induced high-grade tumors were significantly correlated with the degree of intratumoral necrosis (r = 0.63, p < 0.0001) and MVD (r = -0.44, p = 0.008) on pathologic slides. The ADC values were not significantly correlated with tumor cellularity, VEGF expression, or tumor volume in high-grade prostate cancer tissues.
CONCLUSION
In the xenografted prostate cancer model, the ADC values of the solid portion of the tumors are significantly correlated with tumor necrosis and MVD of the pathologic specimens. The ADC values may be utilized as surrogate markers for the noninvasive assessment of tumor necrosis and MVD in high-grade prostate cancer.
MeSH Terms
Figure
Reference
-
1. Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI--a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008. 5:220–233.2. Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009. 11:102–125.3. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007. 188:1622–1635.4. Park MJ, Cha ES, Kang BJ, Ihn YK, Baik JH. The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol. 2007. 8:390–396.5. Kim JK, Jang YJ, Cho G. Multidisciplinary functional MR imaging for prostate cancer. Korean J Radiol. 2009. 10:535–551.6. Heo SH, Jeong YY, Shin SS, Kim JW, Lim HS, Lee JH, et al. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010. 11:295–303.7. Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001. 22:1081–1088.8. Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999. 9:53–60.9. Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol. 1995. 13:765–782.10. Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998. 28:68–77.11. Bulakbasi N, Kocaoglu M, Ors F, Tayfun C, Ucoz T. Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors. AJNR Am J Neuroradiol. 2003. 24:225–233.12. Kim JH, Lim MK, Jeon TY, Rha JH, Eo H, Yoo SY, et al. Diffusion and perfusion characteristics of MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode) in thirteen patients. Korean J Radiol. 2011. 12:15–24.13. Woodhams R, Kakita S, Hata H, Iwabuchi K, Umeoka S, Mountford CE, et al. Diffusion-weighted imaging of mucinous carcinoma of the breast: evaluation of apparent diffusion coefficient and signal intensity in correlation with histologic findings. AJR Am J Roentgenol. 2009. 193:260–266.14. Wang XZ, Wang B, Gao ZQ, Liu JG, Liu ZQ, Niu QL, et al. Diffusion-weighted imaging of prostate cancer: correlation between apparent diffusion coefficient values and tumor proliferation. J Magn Reson Imaging. 2009. 29:1360–1366.15. Zelhof B, Pickles M, Liney G, Gibbs P, Rodrigues G, Kraus S, et al. Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int. 2009. 103:883–888.16. Tan CH, Wang J, Kundra V. Diffusion weighted imaging in prostate cancer. Eur Radiol. 2011. 21:593–603.17. Jenkinson MD, du Plessis DG, Smith TS, Brodbelt AR, Joyce KA, Walker C. Cellularity and apparent diffusion coefficient in oligodendroglial tumours characterized by genotype. J Neurooncol. 2010. 96:385–392.18. Matsumoto Y, Kuroda M, Matsuya R, Kato H, Shibuya K, Oita M, et al. In vitro experimental study of the relationship between the apparent diffusion coefficient and changes in cellularity and cell morphology. Oncol Rep. 2009. 22:641–648.19. Jansen JF, Koutcher JA, Shukla-Dave A. Non-invasive imaging of angiogenesis in head and neck squamous cell carcinoma. Angiogenesis. 2010. 13:149–160.20. Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993. 143:401–409.21. Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, et al. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002. 20:1775–1785.22. Pruneri G, Ponzoni M, Ferreri AJ, Decarli N, Tresoldi M, Raggi F, et al. Microvessel density, a surrogate marker of angiogenesis, is significantly related to survival in multiple myeloma patients. Br J Haematol. 2002. 118:817–820.23. Sun HC, Tang ZY. Angiogenesis in hepatocellular carcinoma: the retrospectives and perspectives. J Cancer Res Clin Oncol. 2004. 130:307–319.24. Lang P, Wendland MF, Saeed M, Gindele A, Rosenau W, Mathur A, et al. Osteogenic sarcoma: noninvasive in vivo assessment of tumor necrosis with diffusion-weighted MR imaging. Radiology. 1998. 206:227–235.25. Vossen JA, Buijs M, Geschwind JF, Liapi E, Prieto Ventura V, Lee KH, et al. Diffusion-weighted and Gd-EOB-DTPA-contrast-enhanced magnetic resonance imaging for characterization of tumor necrosis in an animal model. J Comput Assist Tomogr. 2009. 33:626–630.26. Shinmoto H, Oshio K, Tanimoto A, Higuchi N, Okuda S, Kuribayashi S, et al. Biexponential apparent diffusion coefficients in prostate cancer. Magn Reson Imaging. 2009. 27:355–359.27. Mulkern RV, Barnes AS, Haker SJ, Hung YP, Rybicki FJ, Maier SE, et al. Biexponential characterization of prostate tissue water diffusion decay curves over an extended b-factor range. Magn Reson Imaging. 2006. 24:563–568.28. Klauss M, Lemke A, Grunberg K, Simon D, Re TJ, Wente MN, et al. Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest Radiol. 2011. 46:57–63.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intra-Individual, Inter-Vendor Comparison of Diffusion-Weighted MR Imaging of Upper Abdominal Organs at 3.0 Tesla with an Emphasis on the Value of Normalization with the Spleen
- Assessment of Osteoporosis Based on Changes in SNR and ADC Values on MR Diffusion Weighted Images
- SNR and ADC Changes at Increasing b Values among Patients with Lumbar Vertebral Compression Fracture on 1.5T MR Diffusion Weighted Images
- Value of Diffusion Tensor Imaging of Prostate Cancer: Comparison with Systemic Prostate Biopsy
- Diffusion-Weighted MR Imaging of Spinal Cord Infarction