Korean J Radiol.
2016 Apr;17(2):209-217. 10.3348/kjr.2016.17.2.209.
Intra-Individual, Inter-Vendor Comparison of Diffusion-Weighted MR Imaging of Upper Abdominal Organs at 3.0 Tesla with an Emphasis on the Value of Normalization with the Spleen
- Affiliations
-
- 1Department of Radiology, Chonbuk National University Medical School and Hospital, Jeonju 54907, Korea. pichgo@gmail.com
- 2Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute of Chonbuk National University Hospital, Jeonju 54907, Korea.
- KMID: 2360205
- DOI: http://doi.org/10.3348/kjr.2016.17.2.209
Abstract
OBJECTIVE
To compare the apparent diffusion coefficient (ADC) values of upper abdominal organs with 2 different 3.0 tesla MR systems and to investigate the usefulness of normalization using the spleen.
MATERIALS AND METHODS
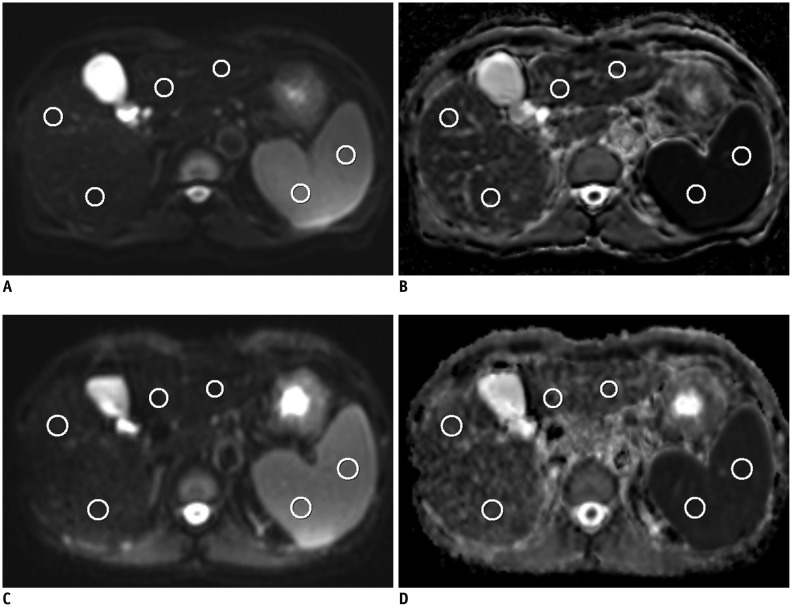
Forty-one patients were enrolled in this prospective study, of which, 35 patients (M:F, 27:8; mean age ± standard deviation, 62.3 ± 12.3 years) were finally analyzed. In addition to the routine liver MR protocol, single-shot spin-echo echo-planar diffusion-weighted imaging using b values of 0, 50, 400, and 800 s/mm2 in 2 different MR systems was performed. ADC values of the liver, spleen, pancreas, kidney and liver lesion (if present) were measured and analyzed. ADC values of the spleen were used for normalization. The Pearson correlation, Spearman correlation, paired sample t test, Wilcoxon signed rank test and Bland-Altman method were used for statistical analysis.
RESULTS
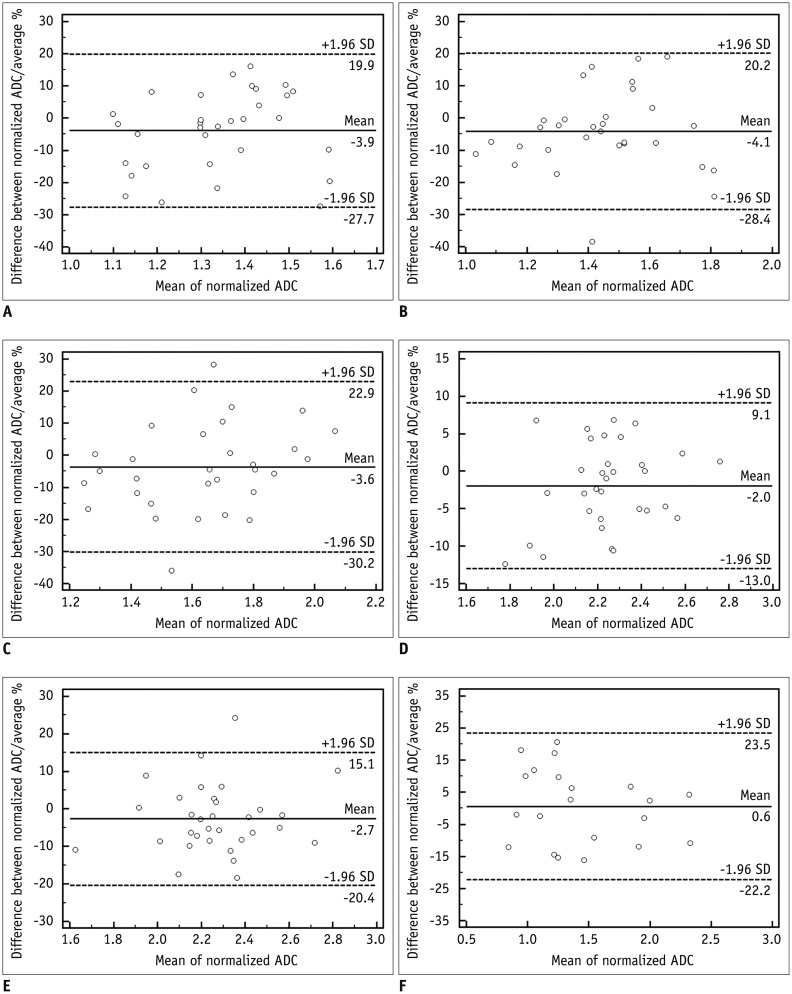
For all anatomical regions and liver lesions, both non-normalized and normalized ADC values from 2 different MR systems showed significant correlations (r = 0.5196-0.8488). Non-normalized ADC values of both MR systems differed significantly in all anatomical regions and liver lesions (p < 0.001). However, the normalized ADC of all anatomical regions and liver lesions did not differ significantly (p = 0.065-0.661), with significantly lower coefficient of variance than that of non-normalized ADC (p < 0.009).
CONCLUSION
Normalization of the abdominal ADC values using the spleen as a reference organ reduces differences between different MR systems, and could facilitate consistent use of ADC as an imaging biomarker for multi-center or longitudinal studies.
MeSH Terms
Figure
Cited by 2 articles
-
Diffusion-Weighted Imaging of Upper Abdominal Organs Acquired with Multiple B-Value Combinations: Value of Normalization Using Spleen as the Reference Organ
Bo Ram Kim, Ji Soo Song, Eun Jung Choi, Seung Bae Hwang, Hong Pil Hwang
Korean J Radiol. 2018;19(3):389-396. doi: 10.3348/kjr.2018.19.3.389.Imaging Findings of Pancreatic Solid Pseudopapillary Neoplasm with High-Grade Malignant Transformation: Focusing on Diffusion-Weighted Imaging and Normalized Apparent Diffusion Coefficient Values
Ka Ram Kang, Ok Ran Shin, Su Lim Lee, Young Mi Ku
J Korean Soc Radiol. 2018;78(3):163-169. doi: 10.3348/jksr.2018.78.3.163.
Reference
-
1. Thoeny HC, De Keyzer F. Extracranial applications of diffusion-weighted magnetic resonance imaging. Eur Radiol. 2007; 17:1385–1393. PMID: 17206421.
Article2. Koh DM, Takahara T, Imai Y, Collins DJ. Practical aspects of assessing tumors using clinical diffusion-weighted imaging in the body. Magn Reson Med Sci. 2007; 6:211–224. PMID: 18239358.
Article3. Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI--a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008; 5:220–233. PMID: 18301415.
Article4. Koinuma M, Ohashi I, Hanafusa K, Shibuya H. Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for evaluation of hepatic fibrosis. J Magn Reson Imaging. 2005; 22:80–85. PMID: 15971188.
Article5. Lewin M, Poujol-Robert A, Boëlle PY, Wendum D, Lasnier E, Viallon M, et al. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007; 46:658–665. PMID: 17663420.
Article6. Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007; 188:1001–1008. PMID: 17377036.
Article7. Low RN, Gurney J. Diffusion-weighted MRI (DWI) in the oncology patient: value of breathhold DWI compared to unenhanced and gadolinium-enhanced MRI. J Magn Reson Imaging. 2007; 25:848–858. PMID: 17335018.
Article8. Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008; 246:812–822. PMID: 18223123.
Article9. Bruegel M, Holzapfel K, Gaa J, Woertler K, Waldt S, Kiefer B, et al. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008; 18:477–485. PMID: 17960390.
Article10. Braithwaite AC, Dale BM, Boll DT, Merkle EM. Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology. 2009; 250:459–465. PMID: 19095786.
Article11. Sasaki M, Yamada K, Watanabe Y, Matsui M, Ida M, Fujiwara S, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology. 2008; 249:624–630. PMID: 18936317.
Article12. Koh DM, Blackledge M, Collins DJ, Padhani AR, Wallace T, Wilton B, et al. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol. 2009; 19:2728–2738. PMID: 19547986.
Article13. Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B. Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. J Magn Reson Imaging. 2011; 33:128–135. PMID: 21182130.
Article14. Zhang JL, Sigmund EE, Chandarana H, Rusinek H, Chen Q, Vivier PH, et al. Variability of renal apparent diffusion coefficients: limitations of the monoexponential model for diffusion quantification. Radiology. 2010; 254:783–792. PMID: 20089719.
Article15. Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009; 11:102–125. PMID: 19186405.
Article16. Donati OF, Chong D, Nanz D, Boss A, Froehlich JM, Andres E, et al. Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology. 2014; 270:454–463. PMID: 24471390.
Article17. Papanikolaou N, Gourtsoyianni S, Yarmenitis S, Maris T, Gourtsoyiannis N. Comparison between two-point and four-point methods for quantification of apparent diffusion coefficient of normal liver parenchyma and focal lesions. Value of normalization with spleen. Eur J Radiol. 2010; 73:305–309. PMID: 19091503.
Article18. Outwater EK, Siegelman ES, Radecki PD, Piccoli CW, Mitchell DG. Distinction between benign and malignant adrenal masses: value of T1-weighted chemical-shift MR imaging. AJR Am J Roentgenol. 1995; 165:579–583. PMID: 7645474.
Article19. Tsushima Y, Ishizaka H, Matsumoto M. Adrenal masses: differentiation with chemical shift, fast low-angle shot MR imaging. Radiology. 1993; 186:705–709. PMID: 8430178.
Article20. Do RK, Chandarana H, Felker E, Hajdu CH, Babb JS, Kim D, et al. Diagnosis of liver fibrosis and cirrhosis with diffusion-weighted imaging: value of normalized apparent diffusion coefficient using the spleen as reference organ. AJR Am J Roentgenol. 2010; 195:671–676. PMID: 20729445.
Article21. Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 5th ed. Boston, MA: Houghton Mifflin;2003.22. Bruix J, Sherman M. American Association for the Study of Liver Diseasess. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022. PMID: 21374666.
Article23. Kim SY, Lee SS, Park B, Kim N, Kim JK, Park SH, et al. Reproducibility of measurement of apparent diffusion coefficients of malignant hepatic tumors: effect of DWI techniques and calculation methods. J Magn Reson Imaging. 2012; 36:1131–1138. PMID: 22777895.
Article24. Corona-Villalobos CP, Pan L, Halappa VG, Bonekamp S, Lorenz CH, Eng J, et al. Agreement and reproducibility of apparent diffusion coefficient measurements of dual-b-value and multi-b-value diffusion-weighted magnetic resonance imaging at 1.5 Tesla in phantom and in soft tissues of the abdomen. J Comput Assist Tomogr. 2013; 37:46–51. PMID: 23321832.
Article25. Miquel ME, Scott AD, Macdougall ND, Boubertakh R, Bharwani N, Rockall AG. In vitro and in vivo repeatability of abdominal diffusion-weighted MRI. Br J Radiol. 2012; 85:1507–1512. PMID: 22674704.26. Yoshikawa T, Kawamitsu H, Mitchell DG, Ohno Y, Ku Y, Seo Y, et al. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006; 187:1521–1530. PMID: 17114546.
Article27. Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999; 210:617–623. PMID: 10207458.
Article28. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010; 254:47–66. PMID: 20032142.
Article29. Kakite S, Dyvorne H, Besa C, Cooper N, Facciuto M, Donnerhack C, et al. Hepatocellular carcinoma: short-term reproducibility of apparent diffusion coefficient and intravoxel incoherent motion parameters at 3.0T. J Magn Reson Imaging. 2015; 41:149–156. PMID: 24415565.
Article30. Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004; 22:275–282. PMID: 15468951.31. Kwee TC, Takahara T, Koh DM, Nievelstein RA, Luijten PR. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging. 2008; 28:1141–1148. PMID: 18972355.
Article32. Chen X, Qin L, Pan D, Huang Y, Yan L, Wang G, et al. Liver diffusion-weighted MR imaging: reproducibility comparison of ADC measurements obtained with multiple breath-hold, free-breathing, respiratory-triggered, and navigator-triggered techniques. Radiology. 2014; 271:113–125. PMID: 24475860.
Article33. Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999; 173:393–398. PMID: 10430143.
Article34. Klasen J, Lanzman RS, Wittsack HJ, Kircheis G, Schek J, Quentin M, et al. Diffusion-weighted imaging (DWI) of the spleen in patients with liver cirrhosis and portal hypertension. Magn Reson Imaging. 2013; 31:1092–1096. PMID: 23731536.
Article35. Kim SY, Lee SS, Byun JH, Park SH, Kim JK, Park B, et al. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology. 2010; 255:815–823. PMID: 20501719.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion-Weighted Imaging of Upper Abdominal Organs Acquired with Multiple B-Value Combinations: Value of Normalization Using Spleen as the Reference Organ
- Diffusion-Weighted MR Imaging of Upper Abdomen: Comparison of Breath-Hold, Free-Breathing, and Respiratory-Triggered Techniques
- Inter-Vendor and Inter-Session Reliability of Diffusion Tensor Imaging: Implications for Multicenter Clinical Imaging Studies
- A Comparison of Lesion Detection and Conspicuity on T2-weighted Images (T2 FFE), FLAIR and Diffusion-weighted Images in Patients with Traumatic Brain Injury
- MR Imaging of Skeletal Muscle Injury in Rabbit: Comparison bet ween Diffusion and T2-weighted MR Images