Korean J Ophthalmol.
2010 Apr;24(2):108-118. 10.3341/kjo.2010.24.2.108.
Retinal Protective Effects of Resveratrol via Modulation of Nitric Oxide Synthase on Oxygen-induced Retinopathy
- Affiliations
-
- 1Department of Pediatrics, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 2Department of Ophthalmology, Dongguk University College of Medicine, Gyeongju, Korea. suksu@dongguk.ac.kr
- KMID: 946003
- DOI: http://doi.org/10.3341/kjo.2010.24.2.108
Abstract
- PURPOSE
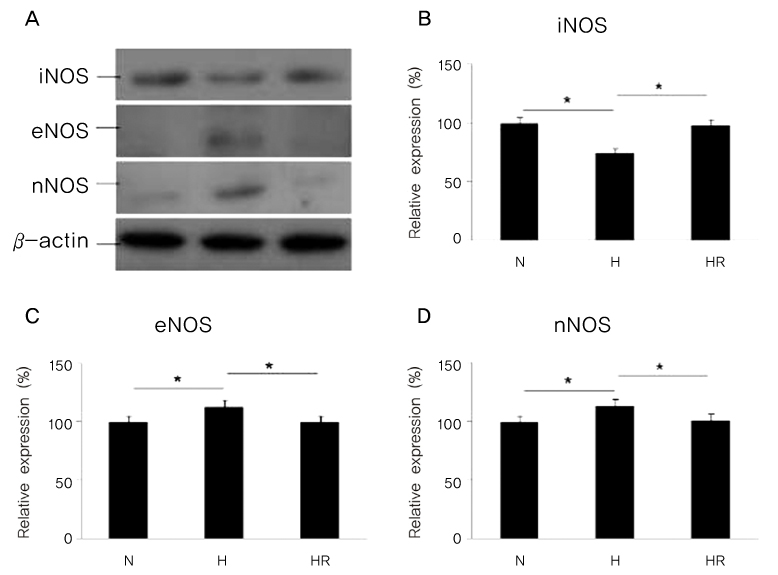
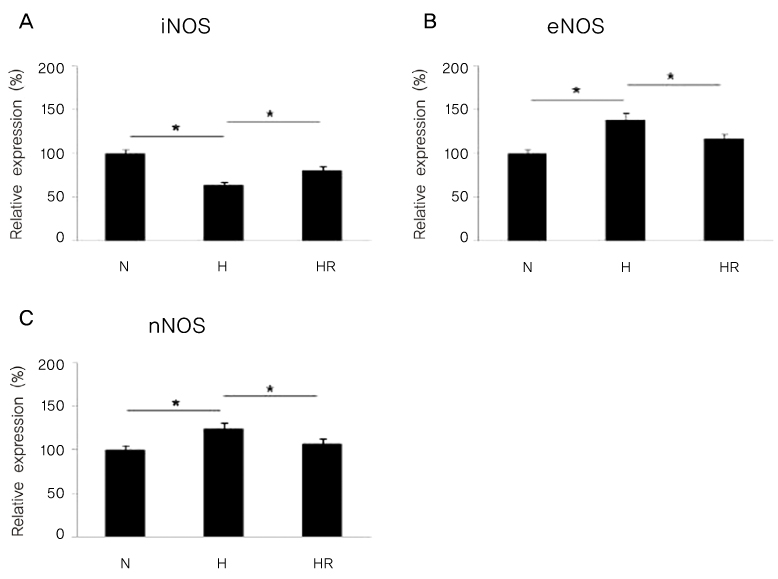
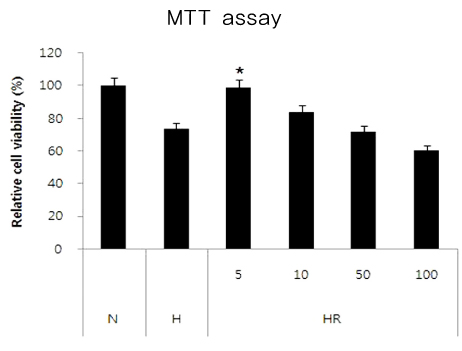
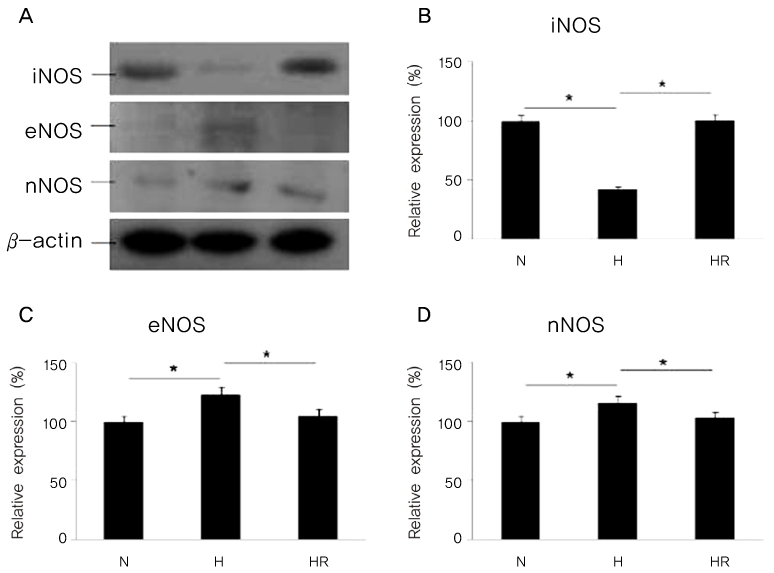
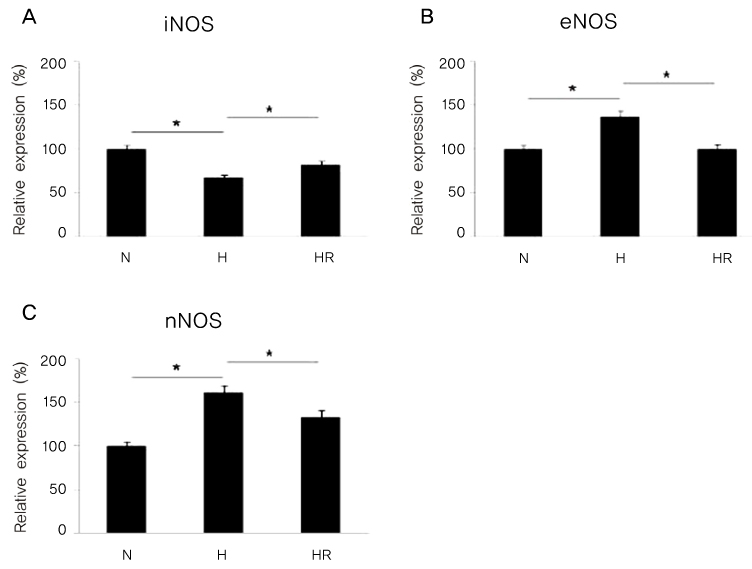
Retinopathy of prematurity (ROP) is one of the leading causes of blindness, with retinal detachment occurring due to oxygen toxicity in preterm infants. Recently, advances in neonatal care have led to improved survival rates for preterm infants, and ROP has increased in incidence. In the present study, we aimed to determine whether or not resveratrol exhibits protective effects in an animal model of ROP and in primary retinal cell cultures of neonatal rat via nitric oxide (NO)-modulating actions using western blotting and real-time PCR with inducible nitric oxide synthase (iNOS), endothelial NOS (eNOS) and neuronal NOS (nNOS) antibodies and mRNAs. METHODS: In an in vivo oxygen-induced retinopathy (OIR) model, cyclic hyperoxia was induced with 80% O2 for one day and 21% O2 for one day from P1 to P14 in newborn Sprague-Dawley (SD) rats. Resveratrol was injected intravitreally for seven days and rats were sacrificed at P21. In vitro OIR primary retinal cell culture was performed using P0-2 SD rats. Hyperoxia injuries were induced through 100% O2 exposure for six hours. Western blotting and real-time PCR using iNOS, eNOS, nNOS antibodies and primers were performed in the rat model of ROP and the dispersed retinal cell culture. RESULTS: In both in vivo and in vitro OIR, the expression of iNOS antibody and mRNA was increased and of eNOS and nNOS were reduced in the resveratrol-treated group. CONCLUSIONS: In conclusion, resveratrol appeared to exert retinal protective effects via modulation of NO-mediated mechanism in in vivo and in vitro OIR models.
MeSH Terms
-
Analysis of Variance
Animals
Animals, Newborn
Blotting, Western
Disease Models, Animal
Electrophoresis, Polyacrylamide Gel
Humans
Infant, Newborn
Nitric Oxide Synthase/*metabolism
Oxygen/toxicity
RNA/metabolism
RNA, Messenger/metabolism
Rats
Rats, Sprague-Dawley
Retina/drug effects/pathology
Retinopathy of Prematurity/*metabolism/pathology/*prevention & control
Reverse Transcriptase Polymerase Chain Reaction
Stilbenes/*pharmacology
Figure
Cited by 1 articles
-
Flow Cytometric Analysis of the Effects of Resveratrol on the Survival of Human Tennon's Capsule Fibroblasts
See Eun Lee, Keun Hae Kim, Jae Woo Kim
J Korean Ophthalmol Soc. 2015;56(8):1268-1273. doi: 10.3341/jkos.2015.56.8.1268.
Reference
-
1. Palmer EA, Flynn JT, Hardy RJ, et al. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991. 98:1628–1640.2. Mantagos IS, Vanderveen DK, Smith LE. Emerging treatments for retinopathy of prematurity. Semin Ophthalmol. 2009. 24:82–86.3. Palmer EA, Hardy RJ, Dobson V, et al. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005. 123:311–318.4. Good WV. Retinopathy of prematurity and the peripheral retina. J Pediatr. 2008. 153:591–592.5. Heidary G, Vanderveen D, Smith LE. Retinopathy of prematurity: current concepts in molecular pathogenesis. Semin Ophthalmol. 2009. 24:77–81.6. Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci. 1993. 34:576–585.7. Seigel GM. Establishment of an E1A-immortalized retinal cell culture. In vitro Cell Dev Biol Anim. 1996. 32:66–68.8. Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997. 11:287–313.9. Creasy LL, Coffee M. Phytoalexin production potential of grape berries. J Am Soc Hortic Sci. 1988. 113:230–234.10. Sun AY, Simonyi A, Sun GY. The "French Paradox" and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med. 2002. 32:314–318.11. Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993. 341:1103–1104.12. Shin NH, Ryu SY, Lee H, et al. Inhibitory effects of hydroxystilbenes on cyclooxygenase from sheep seminal vesicles. Planta Med. 1998. 64:283–284.13. Bertelli AA, Giovannini L, Giannessi D, et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995. 17:1–3.14. Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996. 27:363–366.15. Seo MA, Jang YY, Choi EJ, et al. The expression patterns of nitric oxide synthases (NOS) by resveratrol in hypoxicischemic brain injury in neonatal rat model. Korean J Perinatol. 2008. 19:283–292.16. Lee HJ, Ju M, Park HJ, et al. The expression of nitric oxide synthase (NOS) isoforms in relation to Resveratrol administration in hypoxic injury of myocardial cells. J Korean Pediatr Cardiol Soc. 2007. 11:199–205.17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.18. Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens. Am J Ophthalmol. 1942. 25:203–204.19. Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in premature infants, II: report of cases-clinical aspects. Arch Ophthalmol. 1943. 29:36–53.20. Owens WC, Owens EU. Retrolental fibroplasia in premature infants. Am J Ophthalmol. 1949. 32:1–29.21. Kinsey VE. Retrolental fibroplasia; cooperative study of retrolental fibroplasia and the use of oxygen. AMA Arch Ophthalmol. 1956. 56:481–543.22. Rubaltelli DM, Hirose T. Retinopathy of prematurity update. Int Ophthalmol Clin. 2008. 48:225–235.23. Fleck BW, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev. 2008. 84:83–88.24. Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000. 41:1217–1228.25. Leske DA, Wu J, Fautsch MP, et al. The role of VEGF and IGF-1 in a hypercarbic oxygen-induced retinopathy rat model of ROP. Mol Vis. 2004. 10:43–50.26. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007. 10:133–140.27. Cunningham S, Fleck BW, Elton RA, McIntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995. 346:1464–1465.28. McColm JR, Cunningham S, Wade J, et al. Hypoxic oxygen fluctuations produce less severe retinopathy than hyperoxic fluctuations in a rat model of retinopathy of prematurity. Pediatr Res. 2004. 55:107–113.29. Seigel GM. The golden age of retinal cell culture. Mol Vis. 1999. 5:4.30. Politi LE, Adler R. Generation of enriched populations of cultured photoreceptor cells. Invest Ophthalmol Vis Sci. 1986. 27:656–665.31. Cahill GM, Besharse JC. Light-sensitive melatonin synthesis by Xenopus photoreceptors after destruction of the inner retina. Vis Neurosci. 1992. 8:487–490.32. Wang X, Silverman M. A new technique for isolation of photoreceptor layer. Zhonghua Yan Ke Za Zhi. 1996. 32:304–306.33. Seigel GM, Mutchler AL, Imperato EL. Expression of glial markers in a retinal precursor cell line. Mol Vis. 1996. 2:2.34. Laabich A, Vissvesvaran GP, Lieu KL, et al. Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Invest Ophthalmol Vis Sci. 2006. 47:3156–3163.35. Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993. 329:2002–2012.36. Riddell DR, Owen JS. Nitric oxide and platelet aggregation. Vitam Horm. 1999. 57:25–48.37. Konishi R, Shimizu R, Firestone L, et al. Nitric oxide prevents human platelet adhesion to fiber membranes in whole blood. ASAIO J. 1996. 42:M850–M853.38. Dal Secco D, Paron JA, de Oliveira SH, et al. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide. 2003. 9:153–164.39. Faraci FM, Brian JE Jr. Nitric oxide and the cerebral circulation. Stroke. 1994. 25:692–703.40. Morikawa E, Moskowitz MA, Huang Z, et al. L-arginine infusion promotes nitric oxide-dependent vasodilation, increases regional cerebral blood flow, and reduces infarction volume in the rat. Stroke. 1994. 25:429–435.41. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001. 357:593–615.42. Tsai SK, Hung LM, Fu YT, et al. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007. 46:346–353.43. Rotondo S, Rajtar G, Manarini S, et al. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol. 1998. 123:1691–1699.44. Aggarwal BB, Bhardwaj A, Aggarwal RS, et al. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004. 24:2783–2840.45. Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999. 9:119–131.46. Huang SS, Tsai MC, Chih CL, et al. Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci. 2001. 69:1057–1065.47. Hattori R, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2002. 282:H1988–H1995.48. Chung EY, Kim BH, Lee MK, et al. Anti-inflammatory effect of the oligomeric stilbene alpha-Viniferin and its mode of the action through inhibition of cyclooxygenase-2 and inducible nitric oxide synthase. Planta Med. 2003. 69:710–714.49. Wallerath T, Deckert G, Ternes T, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002. 106:1652–1658.50. Chan MM, Mattiacci JA, Hwang HS, et al. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem Pharmacol. 2000. 60:1539–1548.51. Uchida Y, Yamazaki H, Watanabe S, et al. Enhancement of NF-kappaB activity by resveratrol in cytokine-exposed mesangial cells. Clin Exp Immunol. 2005. 142:76–83.52. King RE, Kent KD, Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem Biol Interact. 2005. 151:143–149.53. Notas G, Nifli AP, Kampa M, et al. Resveratrol exerts its antiproliferative effect on HepG2 hepatocellular carcinoma cells, by inducing cell cycle arrest, and NOS activation. Biochim Biophys Acta. 2006. 1760:1657–1666.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Resveratrol via Mediation of Nitric Oxide Synthase (NOS) on Hypoxic Retinal Injury in Neonatal Rats
- Involvement of Fibronectin in the Migration of Macrophage and Expression of Nitric Oxide Synthase in the BCG induced Inflammatory Sites in Rat Bladder
- Stimulation of macrophage function by S-antigen: production of nitric oxide
- Stimulation of macrophage function by interphotoreceptor retinoid-binding protein: production of nitric oxide
- The Expression of Nitric Oxide Synthase (NOS) Isoforms in relation to Resveratrol Administration in Hypoxic Injury of Myocardial Cells