Ann Lab Med.
2023 Jan;43(1):19-28. 10.3343/alm.2023.43.1.19.
Clinical Usefulness of Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry Method for Low Serum Testosterone Measurement
- Affiliations
-
- 1Department of Laboratory Medicine, GCLabs, Yongin, Korea
- 2Department of Laboratory Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
- KMID: 2551573
- DOI: http://doi.org/10.3343/alm.2023.43.1.19
Abstract
- Background
Mass spectrometry methods exhibit higher accuracy and lower variability than immunoassays at low testosterone concentrations. We developed and validated an ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) assay for quantifying serum total testosterone.
Methods
We used an ExionLC UPLC (Sciex, Framingham, MA, USA) system and a Sciex Triple Quad 6500+ (Sciex) MS/MS system in electrospray ionization and positive ion modes with multiple reaction monitoring transitions to evaluate precision, accuracy, linearity, lower limit of quantitation (LLOQ), carryover, ion suppression, stability, and reference intervals. For method comparison, we measured serum testosterone concentrations using this method in 40 subjects whose testosterone concentrations ranged from 0.14 to 55.48 nmol/L as determined using the Architect i2000 immunoassay (Abbott Diagnostics, Abbott Park, IL, USA) and in an additional 160 sera with testosterone concentrations <1.67 nmol/L.
Results
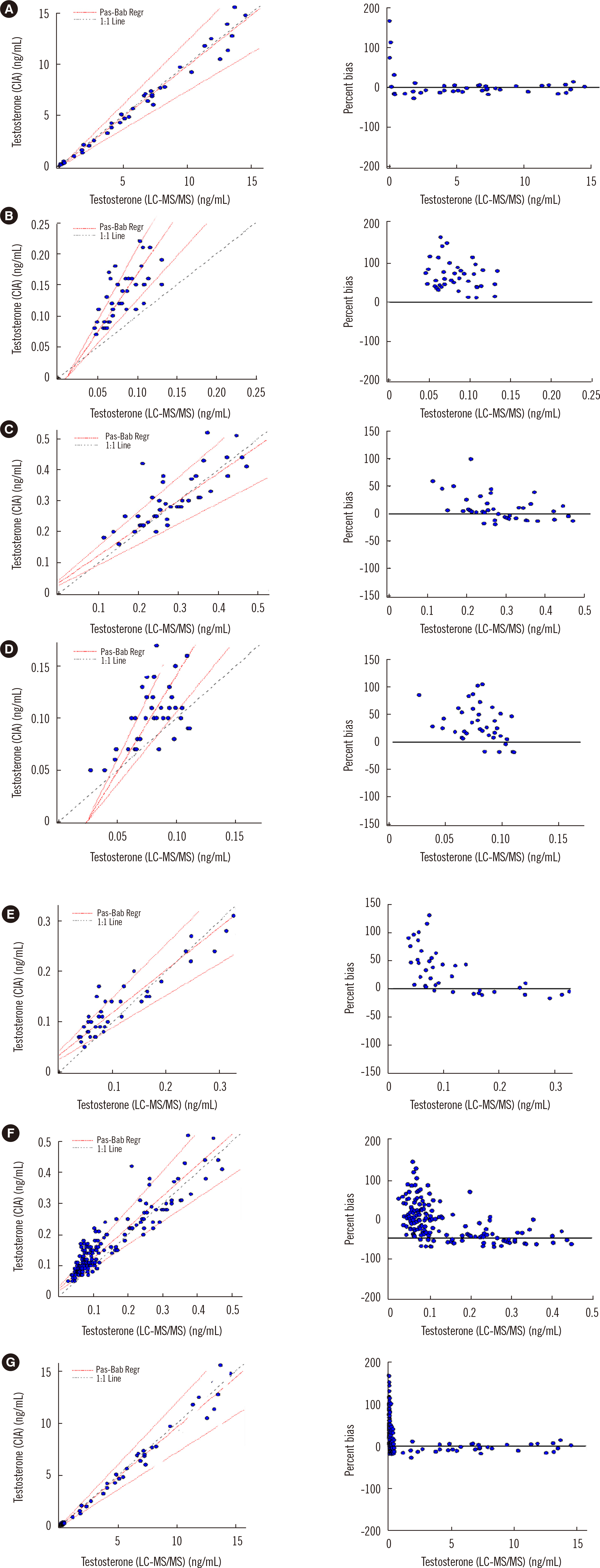
The intra- and inter-run precision CVs were <2.81%, and the accuracy bias values were <3.85%, which were all acceptable. The verified linear interval was 0.03–180.84 nmol/L; the LLOQ was 0.03 nmol/L. No significant carryover and ion suppression were observed. The testosterone in serum was stable at 4°C, at –20°C, and after three freeze-thaw cycles. The reference intervals were successfully verified. The correlation was good at testosterone concentrations of 0.14–55.48 nmol/L; however, the Architect assay showed positive percent bias at concentrations <1.67 nmol/L.
Conclusions
The UPLC-MS/MS assay shows acceptable performance, with a lower LLOQ than the immunoassay. This method will enable the quantitation of low testosterone concentrations.
Figure
Reference
-
1. Zhou H, Wang Y, Gatcombe M, Farris J, Botelho JC, Caudill SP, et al. 2017; Simultaneous measurement of total estradiol and testosterone in human serum by isotope dilution liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 409:5943–54. DOI: 10.1007/s00216-017-0529-x. PMID: 28801832. PMCID: PMC5693763.
Article2. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. 2007; Position statement: utility, limitations, and pitfalls in measuring testosterone: an endocrine society position statement. J Clin Endocrinol Metab. 92:405–13. DOI: 10.1210/jc.2006-1864. PMID: 17090633.3. Zhou Y, Wang S. 2019; A robust LC-MS/MS assay with online cleanup for measurement of serum testosterone. J Sep Sci. 42:2561–8. DOI: 10.1002/jssc.201801189. PMID: 31106475.
Article4. Herold DA, Fitzgerald RL. 2003; Immunoassays for testosterone in women: better than a guess? Clin Chem. 49:1250–1. DOI: 10.1373/49.8.1250. PMID: 12881438.
Article5. Rosner W, Vesper H, editors. Endocrine Society. American Association for Clinical Chemistry. American Association of Clinical Endocrinologists. Androgen Excess/PCOS Society. 2010; Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 95:4542–8. DOI: 10.1210/jc.2010-1314. PMID: 20926540.
Article6. La'ulu SL, Kalp KJ, Straseski JA. 2018; How low can you go? Analytical performance of five automated testosterone immunoassays. Clin Biochem. 58:64–71. DOI: 10.1016/j.clinbiochem.2018.05.008. PMID: 29763574.7. Vesper HW, Botelho JC, Shacklady C, Smith A, Myers GL. 2008; CDC project on standardizing steroid hormone measurements. Steroids. 73:1286–92. DOI: 10.1016/j.steroids.2008.09.008. PMID: 18834895.
Article8. Centers for Disease Control and Prevention, Hormone Standardization Program (CDC HoSt). https://www.cdc.gov/labstandards/hs_standardization.html. Updated on Dec 2021.9. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Bioanalytical method validation guidance for industry. http://www.fda.gov/regulatory-information/serach-fda-guidance-documents/bioanalytical-method-validation-guiodance-industry. Updated on May 2018.10. CLSI. 2014. Liquid chromatography-mass spectrometry methods. 1st ed. C62-A. Clinical and Laboratory Standards Institute;Wayne, PA: DOI: 10.1016/j.steroids.2008.09.008.11. CLSI. 2020. Evaluation of linearity of quantitative measurement procedures. 2nd ed. EP06-ED2. Clinical and Laboratory Standards Institute;Wayne, PA: DOI: 10.1016/j.steroids.2008.09.008.12. French D. 2013; Development and validation of a serum total testosterone liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay calibrated to NIST SRM 971. Clin Chim Acta. 415:109–17. DOI: 10.1016/j.cca.2012.10.007. PMID: 23085208.
Article13. Rappold BA. 2022; Review of the use of liquid chromatography-tandem mass spectrometry in clinical laboratories: Part I-Development. Ann Lab Med. 42:121–40. DOI: 10.3343/alm.2022.42.2.121. PMID: 34635606. PMCID: PMC8548246.
Article14. Rappold BA. 2022; Review of the use of liquid chromatography-tandem mass spectrometry in clinical laboratories: Part II-Operations. Ann Lab Med. 42:531–57. DOI: 10.3343/alm.2022.42.5.531. PMID: 35470272. PMCID: PMC9057814.
Article15. Yun YM, Botelho JC, Chandler DW, Katayev A, Roberts WL, Stanczyk FZ, et al. 2012; Performance criteria for testosterone measurements based on biological variation in adult males: recommendations from the Partnership for the Accurate Testing of Hormones. Clin Chem. 58:1703–10. DOI: 10.1373/clinchem.2012.186569. PMID: 23065474.
Article16. CLSI. 2010. Defining, establishing, and verifying reference intervals in the clinical laboratory. 3rd ed. EP28-A3c. Clinical and Laboratory Standards Institute;Wayne, PA: DOI: 10.1373/clinchem.2012.186569.17. Architect 2nd Generation Testosterone Instruction 2P13 G6-9345/R03 B2P1WZ. www.abbottdiagnostics.com.18. Moal V, Mathieu E, Reynier P, Malthièry Y, Gallois Y. 2007; Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clin Chim Acta. 386:12–9. DOI: 10.1016/j.cca.2007.07.013. PMID: 17706625.
Article19. Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Bunker AM, Fitzgerald RL, et al. 2006; Performance characteristics of a novel tandem mass spectrometry assay for serum testosterone. Clin Chem. 52:120–8. DOI: 10.1373/clinchem.2005.052167. PMID: 16299050.
Article20. McCartney CR, Burt Solorzano CM, Patrie JT, Marshall JC, Haisenleder DJ. 2018; Estimating testosterone concentrations in adolescent girls: comparison of two direct immunoassays to liquid chromatography-tandem mass spectrometry. Steroids. 140:62–9. DOI: 10.1016/j.steroids.2018.09.001. PMID: 30217784. PMCID: PMC7017602.
Article21. Vesper HW, Bhasin S, Wang C, Tai SS, Dodge LA, Singh RJ, et al. 2009; Interlaboratory comparison study of serum total testosterone measurements performed by mass spectrometry methods. Steroids. 74:498–503. DOI: 10.1016/j.steroids.2009.01.004. PMID: 19428438.
Article22. College of American Pathologists, Chemistry Resource Committee, Y-A 2021, Ligand special, participant summary 2021. www.cap.org.23. College of American Pathologists, Accuracy Based Testing Committee, ABS-B 2021, Testosterone and estradiol accuracy, participant summary 2021. www.cap.org.24. Cao ZT, Botelho JC, Rej R, Vesper H. 2017; Accuracy-based proficiency testing for testosterone measurements with immunoassays and liquid chromatography-mass spectrometry. Clin Chim Acta. 469:31–6. DOI: 10.1016/j.cca.2017.03.010. PMID: 28288785. PMCID: PMC5695555.
Article25. Choi R, Park HD, Oh HJ, Lee K, Song J, Lee SY. 2019; Dried blood spot multiplexed steroid profiling using liquid chromatography tandem mass spectrometry in Korean neonates. Ann Lab Med. 39:263–70. DOI: 10.3343/alm.2019.39.3.263. PMID: 30623618. PMCID: PMC6340850.
Article26. Ko DH, Lee K, Jeon SH, Song SH, Yun YM, Chun S, et al. 2016; Simultaneous measurement of serum chemical castration agents and testosterone levels using ultra-performance liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 40:294–303. DOI: 10.1093/jat/bkw017. PMID: 26989223.
Article27. Olisov D, Lee K, Jun SH, Song SH, Kim JH, Lee YA, et al. 2019; Measurement of serum steroid profiles by HPLC-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 1117:1–9. DOI: 10.1016/j.jchromb.2019.04.001. PMID: 30986707.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneously Determining Meropenem and Linezolid in Blood and Cerebrospinal Fluid
- Metabolism and excretion of novel pulmonary-targeting docetaxel liposome in rabbits
- An Accurate Isotope Dilution Liquid Chromatography-Tandem Mass Spectrometry Method for Serum C-Peptide and Its Use in Harmonization in China
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry
- Implementation and Validation of an Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry Method for Quantifying Levetiracetam and Lamotrigine in Serum Specimens