Ann Lab Med.
2023 Jul;43(4):345-354. 10.3343/alm.2023.43.4.345.
An Accurate Isotope Dilution Liquid Chromatography-Tandem Mass Spectrometry Method for Serum C-Peptide and Its Use in Harmonization in China
- Affiliations
-
- 1National Center for Clinical Laboratories, Beijing Engineering Research Center of Laboratory Medicine, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Chinese Academy of Medical Sciences and Peking Union Medical College, China
- 2National Center for Clinical Laboratories, Beijing Engineering Research Center of Laboratory Medicine, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, China
- KMID: 2551952
- DOI: http://doi.org/10.3343/alm.2023.43.4.345
Abstract
- Background
Serum C-peptide results from various routine methods used in China are highly variable, warranting well-performing methods to serve as an accuracy base to improve the harmonization of C-peptide measurements in China. We developed an accurate isotope dilution liquid chromatography-tandem mass spectrometry (ID-LC–MS/MS) method for serum C-peptide measurement and explored its use in harmonization.
Methods
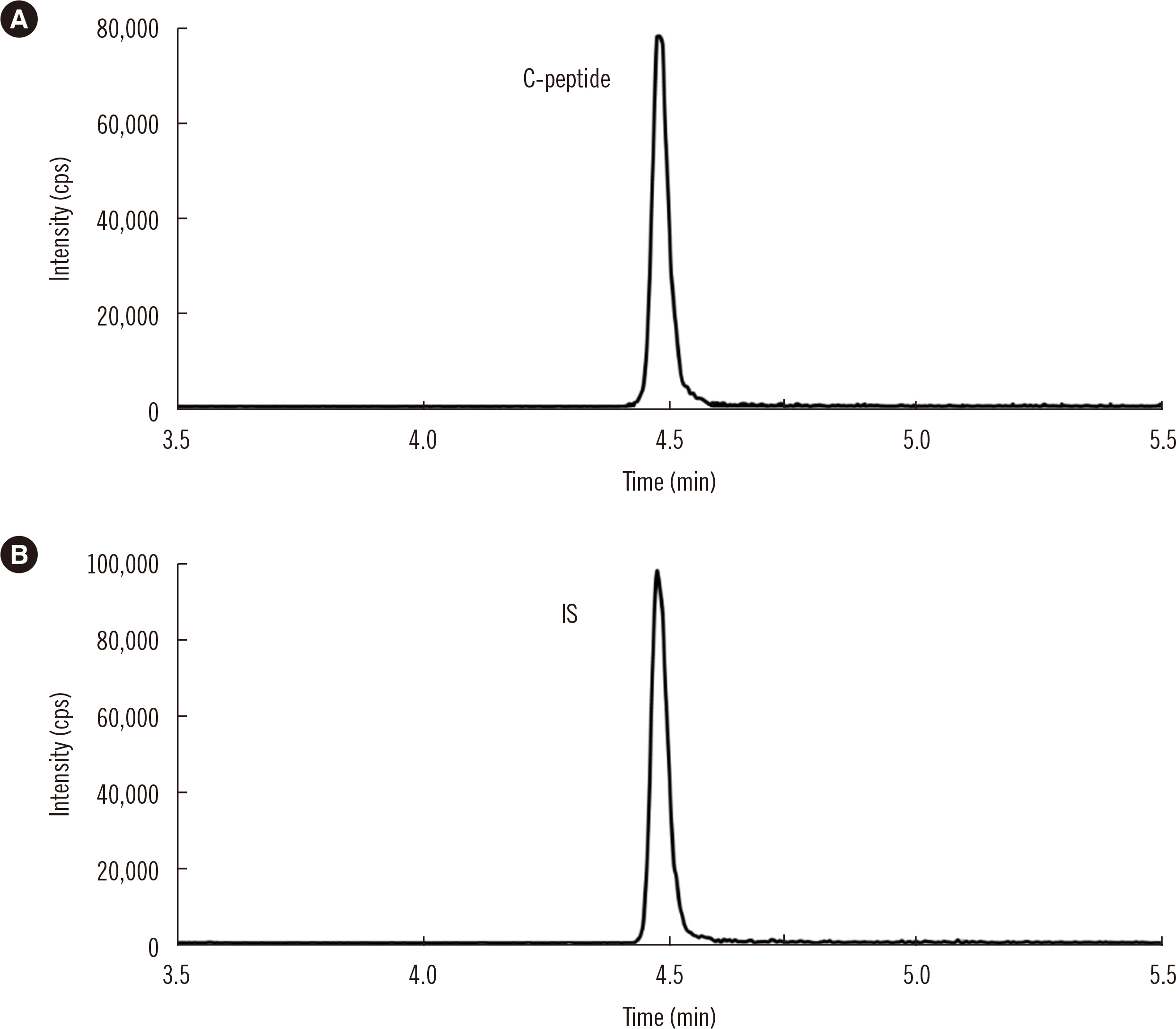
After protein precipitation with ZnSO4 solution, C-peptide was extracted from serum samples by anion-exchange solid-phase extraction and quantified by ID-LC–MS/MS in positive ion mode. The precision and analytical recovery of the ID-LC–MS/MS method were assessed. Seventy-six serum samples were analyzed using the ID-LC–MS/MS method and six routine immunoassays. Ordinary linear regression (OLR) and Bland-Altman (BA) analyses were conducted to evaluate the relationship between the ID-LC–MS/MS method and routine immunoassays. Five serum pool samples assigned using the ID-LC–MS/MS method were used to recalibrate the routine assays. OLR and BA analyses were re-conducted after recalibration.
Results
The within-run, between-run, and total precision for the ID-LC–MS/MS method at four concentrations were 1.0%–2.1%, 0.6%–1.2%, and 1.3%–2.2%, respectively. The analytical recoveries for the ID-LC–MS/MS method at three concentrations were 100.3%–100.7%, 100.4%–101.0%, and 99.6%–100.7%. The developed method and the immunoassays were strongly correlated, with all R2 >0.98. The comparability among the immunoassays was substantially improved after recalibration.
Conclusions
The performance of the ID-LC–MS/MS method was carefully validated, and this method can be used to improve the harmonization of serum C-peptide measurements in China.
Keyword
Figure
Reference
-
1. Zimmet P, Alberti KG, Magliano DJ, Bennett PH. 2016; Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 12:616–22. DOI: 10.1038/nrendo.2016.105. PMID: 27388988.
Article2. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. 2014; Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 103:137–49. DOI: 10.1016/j.diabres.2013.11.002. PMID: 24630390.
Article3. Leighton E, Sainsbury CA, Jones GC. 2017; A practical review of C-peptide testing in diabetes. Diabetes Ther. 8:475–87. DOI: 10.1007/s13300-017-0265-4. PMID: 28484968. PMCID: PMC5446389.
Article4. Jones AG, Hattersley AT. 2013; The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 30:803–17. DOI: 10.1111/dme.12159. PMID: 23413806. PMCID: PMC3748788.
Article5. Oyer PE, Cho S, Peterson JD, Steiner DF. 1971; Studies on human proinsulin. Isolation and amino acid sequence of the human pancreatic C-peptide. J Biol Chem. 246:1375–86. DOI: 10.1016/S0021-9258(19)76984-1. PMID: 5101771.6. Zavaroni I, Deferrari G, Lugari R, Bonora E, Garibotto G, Dall'Aglio E, et al. 1987; Renal metabolism of C-peptide in man. J Clin Endocrinol Metab. 65:494–8. DOI: 10.1210/jcem-65-3-494. PMID: 3624411.
Article7. Henriksen JH, Tronier B, Bülow JB. 1987; Kinetics of circulating endogenous insulin, C-peptide, and proinsulin in fasting nondiabetic man. Metabolism. 36:463–8. DOI: 10.1016/0026-0495(87)90044-8. PMID: 3553849.
Article8. Zhou W, Deng Y, Zhao H, Zhang C. 2022; Current status of serum insulin and C-peptide measurement in clinical laboratories: experience from 94 laboratories in China. Ann Lab Med. 42:428–37. DOI: 10.3343/alm.2022.42.4.428. PMID: 35177563. PMCID: PMC8859554.
Article9. Little RR, Wielgosz RI, Josephs R, Kinumi T, Takatsu A, Li H, et al. 2017; Implementing a reference measurement system for C-peptide: successes and lessons learned. Clin Chem. 63:1447–56. DOI: 10.1373/clinchem.2016.269274. PMID: 28646033. PMCID: PMC5575958.
Article10. Liu Z, Liu Q, Deng Y, Zhao H, Zeng J, Zhang T, et al. 2021; Quantitation of plasma metanephrines using isotope dilution liquid chromatography tandem mass spectrometry (ID-LC/MS/MS): a candidate reference measurement procedure and its application to evaluating routine ID-LC/MS/MS methods. Anal Bioanal Chem. 413:7509–20. DOI: 10.1007/s00216-021-03715-8. PMID: 34643770.
Article11. Kinumi T, Mizuno R, Takatsu A. Quantification of serum C-peptide by isotope-dilution liquid chromatography-tandem mass spectrometry: enhanced detection using chemical modification and immunoaffinity purification. J Chromatogr B Analyt Technol Biomed Life Sci. 2014; 953-4:138–42. DOI: 10.1016/j.jchromb.2014.02.019. PMID: 24607695.
Article12. Kinumi T, Goto M, Eyama S, Kato M, Kasama T, Takatsu A. 2012; Development of SI-traceable C-peptide certified reference material NMIJ CRM 6901-a using isotope-dilution mass spectrometry-based amino acid analyses. Anal Bioanal Chem. 404:13–21. DOI: 10.1007/s00216-012-6097-1. PMID: 22610603.
Article13. CLSI. 2014. User verification of precision and estimation of bias; approved guideline. 3th ed. EP15-A3. Clinical and Laboratory Standards Institute;Wayne, PA:14. ISO/IEC. 2008. Guide 98-3:2008. Uncertainty of measurement-part 3: guide to the expression of uncertainty in measurement. International Organization for Standardization;Geneva:15. CLSI. 2013. Measurement procedure comparison and bias estimation using patient samples; approved guideline. 3th ed. EP09-A3. Clinical and Laboratory Standards Institute;Wayne, PA:16. Stoyanov AV, Connolly S, Rohlfing CL, Rogatsky E, Stein D, Little RR. 2013; Human C-peptide quantitation by LC-MS isotope-dilution assay in serum or urine samples. J Chromatogr Sep Tech. 4:172. DOI: 10.4172/2157-7064.1000172. PMID: 31942247. PMCID: PMC6961834.17. Stoyanov AV, Rohlfing CL, Connolly S, Roberts ML, Nauser CL, Little RR. 2011; Use of cation exchange chromatography for human C-peptide isotope dilution-mass spectrometric assay. J Chromatogr A. 1218:9244–9. DOI: 10.1016/j.chroma.2011.10.080. PMID: 22098929. PMCID: PMC5089808.18. Rogatsky E, Balent B, Goswami G, Tomuta V, Jayatillake H, Cruikshank G, et al. 2006; Sensitive quantitative analysis of C-peptide in human plasma by 2-dimensional liquid chromatography-mass spectrometry isotope-dilution assay. Clin Chem. 52:872–9. DOI: 10.1373/clinchem.2005.063081. PMID: 16556683.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and Validation of a Novel Isotope Dilution-Ultraperformance Liquid ChromatographyTandem Mass Spectrometry Method for Serum C-Peptide
- Comparison of Six Automated Immunoassays With Isotope-Diluted Liquid Chromatography-Tandem Mass Spectrometry for Total Thyroxine Measurement
- A Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneously Determining Meropenem and Linezolid in Blood and Cerebrospinal Fluid
- Metabolism and excretion of novel pulmonary-targeting docetaxel liposome in rabbits
- Sporozoite proteome analysis of Cryptosporidium parvum by one-dimensional SDS-PAGE and liquid chromatography tandem mass spectrometry