Cancer Res Treat.
2024 Jan;56(1):70-80. 10.4143/crt.2023.482.
Tumor Microenvironment Modulation by Neoadjuvant Erlotinib Therapy and Its Clinical Impact on Operable EGFR-Mutant Non–Small Cell Lung Cancer
- Affiliations
-
- 1Center for Lung Cancer, Division of Hematology and Oncology, Department of Internal Medicine, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 2Research Institute, National Cancer Center, Goyang, Korea
- 3Center for Lung Cancer, Department of Thoracic Surgery, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 4Department of Radiology, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 5Department of Pathology, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- KMID: 2550324
- DOI: http://doi.org/10.4143/crt.2023.482
Abstract
- Purpose
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors have greatly improved survival in EGFR-mutant (EGFRm) non–small cell lung cancer (NSCLC); however, their effects on the tumor microenvironment (TME) are unknown. We assessed the changes induced by neoadjuvant erlotinib therapy (NE) in the TME of operable EGFRm NSCLC.
Materials and Methods
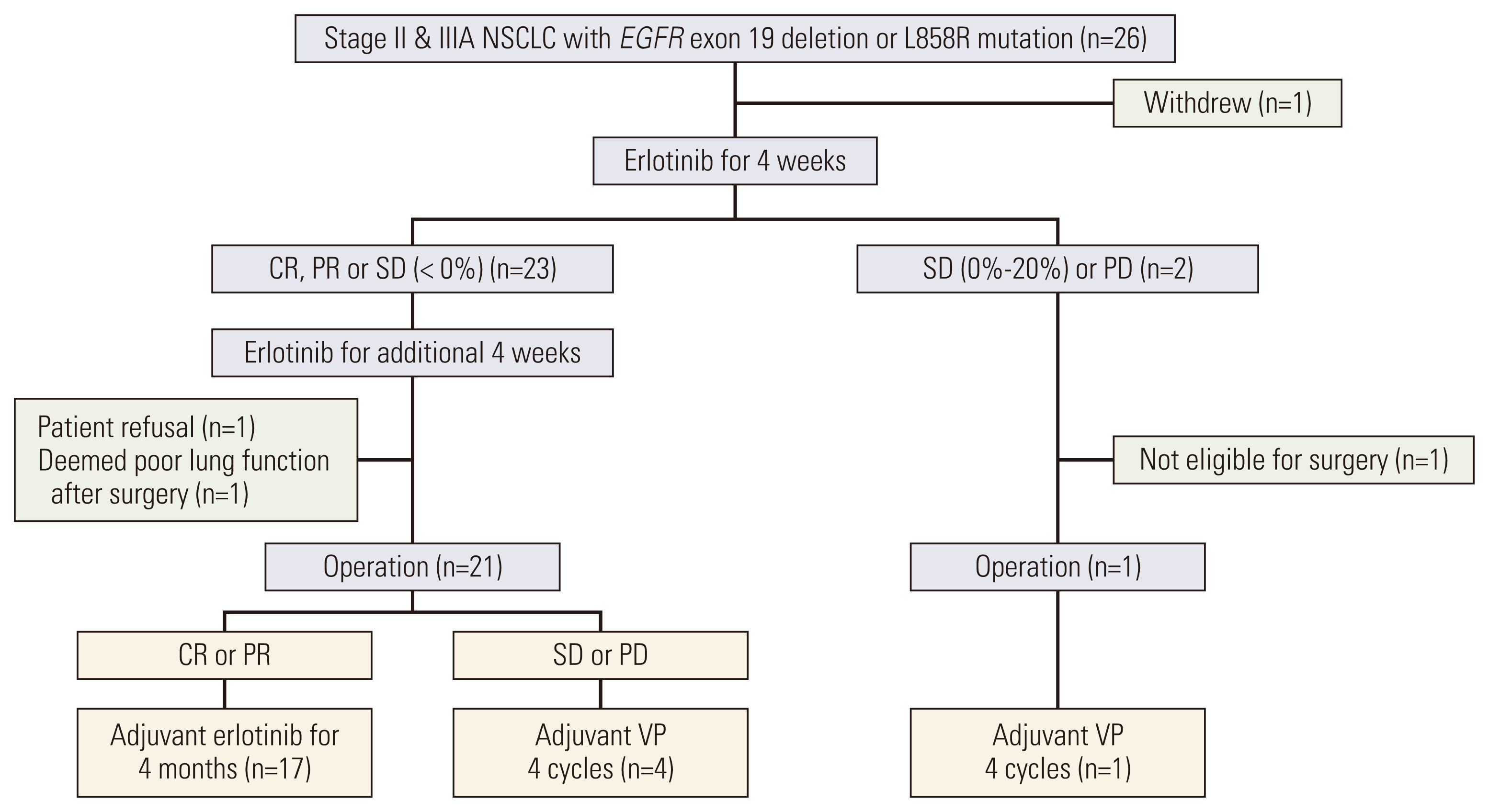
This was a single-arm phase II trial for neoadjuvant/adjuvant erlotinib therapy in patients with stage II/IIIA EGFRm NSCLC (EGFR exon 19 deletion or L858R mutations). Patients received up to 2 cycles of NE (150 mg/day) for 4 weeks, followed by surgery and adjuvant erlotinib or vinorelbine plus cisplatin therapy depending on observed NE response. TME changes were assessed based on gene expression analysis and mutation profiling.
Results
A total of 26 patients were enrolled; the median age was 61, 69% were female, 88% were stage IIIA, and 62% had L858R mutation. Among 25 patients who received NE, the objective response rate was 72% (95% confidence interval [CI], 52.4 to 85.7). The median disease-free and overall survival (OS) were 17.9 (95% CI, 10.5 to 25.4) and 84.7 months (95% CI, 49.7 to 119.8), respectively. Gene set enrichment analysis in resected tissues revealed upregulation of interleukin, complement, cytokine, transforming growth factor β, and hedgehog pathways. Patients with upregulated pathogen defense, interleukins, and T-cell function pathways at baseline exhibited partial response to NE and longer OS. Patients with upregulated cell cycle pathways at baseline exhibited stable/progressive disease after NE and shorter OS.
Conclusion
NE modulated the TME in EGFRm NSCLC. Upregulation of immune-related pathways was associated with better outcomes.
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.2. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.3. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.4. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.5. Yue D, Xu S, Wang Q, Li X, Shen Y, Zhao H, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med. 2018; 6:863–73.6. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Wei YC, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol. 2021; 39:713–22.7. Zhong WZ, Chen KN, Chen C, Gu CD, Wang J, Yang XN, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019; 37:2235–45.8. Bruno D, Dowlati A. Immunotherapy in EGFR mutant non-small cell lung cancer: when, who and how? Transl Lung Cancer Res. 2019; 8:710–4.9. Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015; 10:910–23.10. Lin A, Wei T, Meng H, Luo P, Zhang J. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer. 2019; 18:139.11. Jia Y, Li X, Jiang T, Zhao S, Zhao C, Zhang L, et al. EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: Implications for combination therapies. Int J Cancer. 2019; 145:1432–44.12. Chen S, Tang J, Liu F, Li W, Yan T, Shangguan D, et al. Changes of tumor microenvironment in non-small cell lung cancer after TKI treatments. Front Immunol. 2023; 14:1094764.13. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008; 26:3552–9.14. NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014; 383:1561–71.15. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020; 383:1711–23.16. Xiong L, Li R, Sun J, Lou Y, Zhang W, Bai H, et al. Erlotinib as neoadjuvant therapy in stage IIIA (N2) EGFR mutation-positive non-small cell lung cancer: a prospective, single-arm, phase II study. Oncologist. 2019; 24:157.17. Xiong L, Lou Y, Bai H, Li R, Xia J, Fang W, et al. Efficacy of erlotinib as neoadjuvant regimen in EGFR-mutant locally advanced non-small cell lung cancer patients. J Int Med Res. 2020; 48:300060519887275.18. Zhang Y, Fu F, Hu H, Wang S, Li Y, Hu H, et al. Gefitinib as neoadjuvant therapy for resectable stage II-IIIA non-small cell lung cancer: a phase II study. J Thorac Cardiovasc Surg. 2021; 161:434–42.19. Liu S, Ren J, Ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. 2021; 6:8.20. Zhang M, Zhang YY, Chen Y, Wang J, Wang Q, Lu H. TGF-β signaling and resistance to cancer therapy. Front Cell Dev Biol. 2021; 9:786728.21. Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, et al. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. 2019; 129:5151–62.22. Bai XY, Zhang XC, Yang SQ, An SJ, Chen ZH, Su J, et al. Blockade of Hedgehog signaling synergistically increases sensitivity to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer cell lines. PLoS One. 2016; 11:e0149370.23. Ito T, Nagashima H, Akiyama M, Utsumi Y, Sato H, Chiba S, et al. Treatment with immune checkpoint inhibitors after EGFR-TKIs in EGFR-mutated lung cancer. Thorac Cancer. 2022; 13:386–93.24. Lau SK, Boutros PC, Pintilie M, Blackhall FH, Zhu CQ, Strumpf D, et al. Three-gene prognostic classifier for early-stage non small-cell lung cancer. J Clin Oncol. 2007; 25:5562–9.25. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999; 284:770–6.26. Chen B, Li H, Liu C, Wang S, Zhang F, Zhang L, et al. Potential prognostic value of delta-like protein 3 in small cell lung cancer: a meta-analysis. World J Surg Oncol. 2020; 18:226.27. Kolev V, Mandinova A, Guinea-Viniegra J, Hu B, Lefort K, Lambertini C, et al. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008; 10:902–11.28. Arasada RR, Amann JM, Rahman MA, Huppert SS, Carbone DP. EGFR blockade enriches for lung cancer stem-like cells through Notch3-dependent signaling. Cancer Res. 2014; 74:5572–84.29. Zou B, Zhou XL, Lai SQ, Liu JC. Notch signaling and non-small cell lung cancer. Oncol Lett. 2018; 15:3415–21.30. Wei Y, Ren X, Galbo PM Jr, Moerdler S, Wang H, Sica RA, et al. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci Immunol. 2021; 6:eabf9792.31. Bhatt RS, Berjis A, Konge JC, Mahoney KM, Klee AN, Freeman SS, et al. KIR3DL3 is an inhibitory receptor for HHLA2 that mediates an alternative immunoinhibitory pathway to PD1. Cancer Immunol Res. 2021; 9:156–69.32. Xiao Y, Freeman GJ. A New B7:CD28 family checkpoint target for cancer immunotherapy: HHLA2. Clin Cancer Res. 2015; 21:2201–3.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incorporating Erlotinib or Irinotecan Plus Cisplatin into Chemoradiotherapy for Stage III Non-small Cell Lung Cancer According to EGFR Mutation Status
- Efficacy and Safety of Afatinib for EGFR-mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib
- The Role of Brain Radiotherapy before First-Line Afatinib Therapy, Compared to Gefitinib or Erlotinib, in Patients with EGFR-Mutant Non–Small Cell Lung Cancer
- Ovarian Metastasis from Non-Small Cell Lung Cancer Responding to Erlotinib
- Efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors for patient with leptomeningeal metastasis of epidermal growth factor receptor mutant non-small cell lung cancer