Cancer Res Treat.
2017 Oct;49(4):981-989. 10.4143/crt.2016.522.
Incorporating Erlotinib or Irinotecan Plus Cisplatin into Chemoradiotherapy for Stage III Non-small Cell Lung Cancer According to EGFR Mutation Status
- Affiliations
-
- 1Center for Lung Cancer, National Cancer Center, Goyang, Korea. jslee@ncc.re.kr
- 2Center for Proton Therapy, National Cancer Center, Goyang, Korea.
- 3Center for Clinical Trials, National Cancer Center, Goyang, Korea.
- KMID: 2394817
- DOI: http://doi.org/10.4143/crt.2016.522
Abstract
- PURPOSE
Concurrent chemoradiotherapy (CCRT) is the standard care for stage III non-small cell lung cancer (NSCLC) patients; however, a more effective regimen is needed to improve the outcome by better controlling occult metastases. We conducted two parallel randomized phase II studies to incorporate erlotinib or irinotecan-cisplatin (IP) into CCRT for stage III NSCLC depending on epidermal growth factor receptor (EGFR) mutation status.
MATERIALS AND METHODS
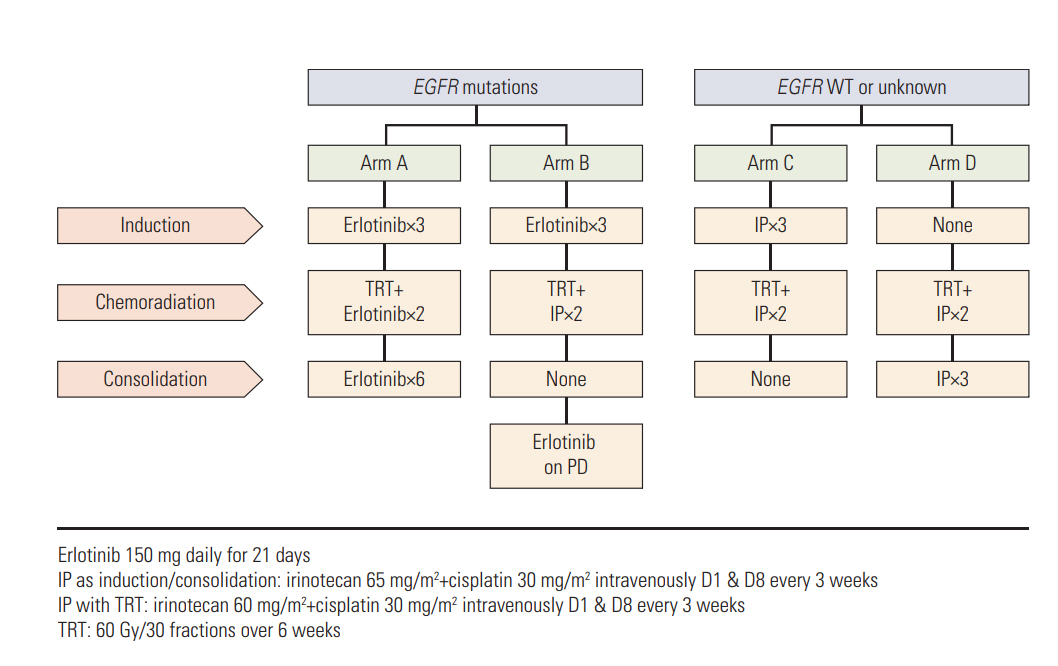
Patients with EGFR-mutant tumors were randomized to receive three cycles of erlotinib first and then either CCRT with erlotinib followed by erlotinib (arm A) or CCRT with IP only (arm B). Patients with EGFR unknown or wild-type tumors were randomized to receive either three cycles of IP before (arm C) or after CCRT with IP (arm D).
RESULTS
Seventy-three patients were screened and the study was closed early because of slow accrual after 59 patients were randomized. Overall, there were seven patients in arm A, five in arm B, 22 in arm C, and 25 in arm D. The response rate was 71.4% and 80.0% for arm A and B, and 70.0% and 73.9% for arm C and D. The median overall survival (OS) was 39.3 months versus 31.2 months for arm A and B (p=0.442), and 16.3 months versus 25.3 months for arm C and D (p=0.050). Patients with sensitive EGFR mutations had significantly longer OS than EGFR-wild patients (74.8 months vs. 25.3 months, p=0.034). There were no unexpected toxicities.
CONCLUSION
Combined-modality treatment by molecular diagnostics is feasible in stage III NSCLC. EGFR-mutant patients appear to be a distinct subset with longer survival.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancerstatistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.3. Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010; 5:29–33.
Article4. Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011; 103:1452–60.
Article5. Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999; 17:2692–9.
Article6. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-smallcell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005; 366:1527–37.
Article7. Tamura K, Okamoto I, Kashii T, Negoro S, Hirashima T, Kudoh S, et al. Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJTOG0403). Br J Cancer. 2008; 98:907–14.
Article8. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.
Article9. Masuda N, Fukuoka M, Fujita A, Kurita Y, Tsuchiya S, Nagao K, et al. A phase II trial of combination of CPT-11 and cisplatin for advanced non-small-cell lung cancer. CPT-11 Lung Cancer Study Group. Br J Cancer. 1998; 78:251–6.10. Mori K, Machida S, Yoshida T, Yoshida M, Kano Y, Tominaga K. A phase II study of irinotecan and infusional cisplatin with recombinant human granulocyte colony-stimulating factor support for advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 1999; 43:467–70.
Article11. Ueoka H, Tanimoto M, Kiura K, Tabata M, Takigawa N, Segawa Y, et al. Fractionated administration of irinotecan and cisplatin for treatment of non-small-cell lung cancer: a phase II study of Okayama Lung Cancer Study Group. Br J Cancer. 2001; 85:9–13.
Article12. Jagasia MH, Langer CJ, Johnson DH, Yunus F, Rodgers JS, Schlabach LL, et al. Weekly irinotecan and cisplatin in advanced non-small cell lung cancer: a multicenter phase II study. Clin Cancer Res. 2001; 7:68–73.13. Han JY, Lim HS, Lee DH, Ju SY, Lee SY, Kim HY, et al. Randomized Phase II study of two opposite administration sequences of irinotecan and cisplatin in patients with advanced nonsmall cell lung carcinoma. Cancer. 2006; 106:873–80.
Article14. Hanna N, Bunn PA Jr, Langer C, Einhorn L, Guthrie T Jr, Beck T, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006; 24:2038–43.
Article15. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.16. Lee DH, Lee GK, Kong SY, Kook MC, Yang SK, Park SY, et al. Epidermal growth factor receptor status in anaplastic thyroid carcinoma. J Clin Pathol. 2007; 60:881–4.
Article17. Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006; 118:257–62.
Article18. Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012; 30:1122–8.
Article19. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as firstline treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.20. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-smallcell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–42.
Article21. Cox JD, Scott CB, Byhardt RW, Emami B, Russell AH, Fu KK, et al. Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non-small cell carcinoma of lung (NSCCL): analysis of radiation therapy oncology group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1999; 43:505–9.
Article22. Andre F, Grunenwald D, Pujol JL, Girard P, Dujon A, Brouchet L, et al. Patterns of relapse of N2 nonsmall-cell lung carcinoma patients treated with preoperative chemotherapy: should prophylactic cranial irradiation be reconsidered? Cancer. 2001; 91:2394–400.23. Law A, Karp DD, Dipetrillo T, Daly BT. Emergence of increased cerebral metastasis after high-dose preoperative radiotherapy with chemotherapy in patients with locally advanced nonsmall cell lung carcinoma. Cancer. 2001; 92:160–4.
Article24. Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT 3rd, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995; 13:1880–92.
Article25. Qi WX, Sun YJ, Shen Z, Yao Y. Risk of interstitial lung disease associated with EGFR-TKIs in advanced non-small-cell lung cancer: a meta-analysis of 24 phase III clinical trials. J Chemother. 2015; 27:40–51.
Article26. Niho S, Ohe Y, Ishikura S, Atagi S, Yokoyama A, Ichinose Y, et al. Induction chemotherapy followed by gefitinib and concurrent thoracic radiotherapy for unresectable locally advanced adenocarcinoma of the lung: a multicenter feasibility study (JCOG 0402). Ann Oncol. 2012; 23:2253–8.
Article27. Komaki R, Allen PK, Wei X, Blumenschein GR, Tang X, Lee JJ, et al. Adding erlotinib to chemoradiation improves overall survival but not progression-free survival in stage III nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys. 2015; 92:317–24.
Article28. Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005; 23:5883–91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- EGFR Mutation–Positive Unresectable Stage III Non-Squamous Lung Cancer Is Associated with a High Incidence of Brain Metastasis
- EGFR Mutation Is Associated with Short Progression-Free Survival in Patients with Stage III Non-squamous Cell Lung Cancer Treated with Concurrent Chemoradiotherapy
- Chemotherapy for Small Cell Lung Cancer
- Treatment of Small Cell Lung Cancer
- Ovarian Metastasis from Non-Small Cell Lung Cancer Responding to Erlotinib