Cancer Res Treat.
2022 Jul;54(3):782-792. 10.4143/crt.2021.843.
Selection Strategies and Practical Application of BRAF V600E-Mutated Non–Small Cell Lung Carcinoma
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Samsung Advanced Institute of Health Science and Technology, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Laboratory of Cancer Genomics and Molecular Pathology, Samsung Medical Center, Seoul, Korea
- 4Research Institute of Pharmaceutical Sciences and College of Pharmacy, Seoul National University, Seoul, Korea
- 5Bio-MAX, Seoul National University, Seoul, Korea
- KMID: 2531324
- DOI: http://doi.org/10.4143/crt.2021.843
Abstract
- Purpose
The incidence of BRAF V600E mutation in non-small cell lung carcinoma (NSCLC) is lower than 2%, which poses difficulties in finding legitimate patients for targeted therapy. We investigated the predictive factors pertaining to BRAF V600E and the effectiveness of the VE1 antibody as a screening method for patient selection.
Materials and Methods
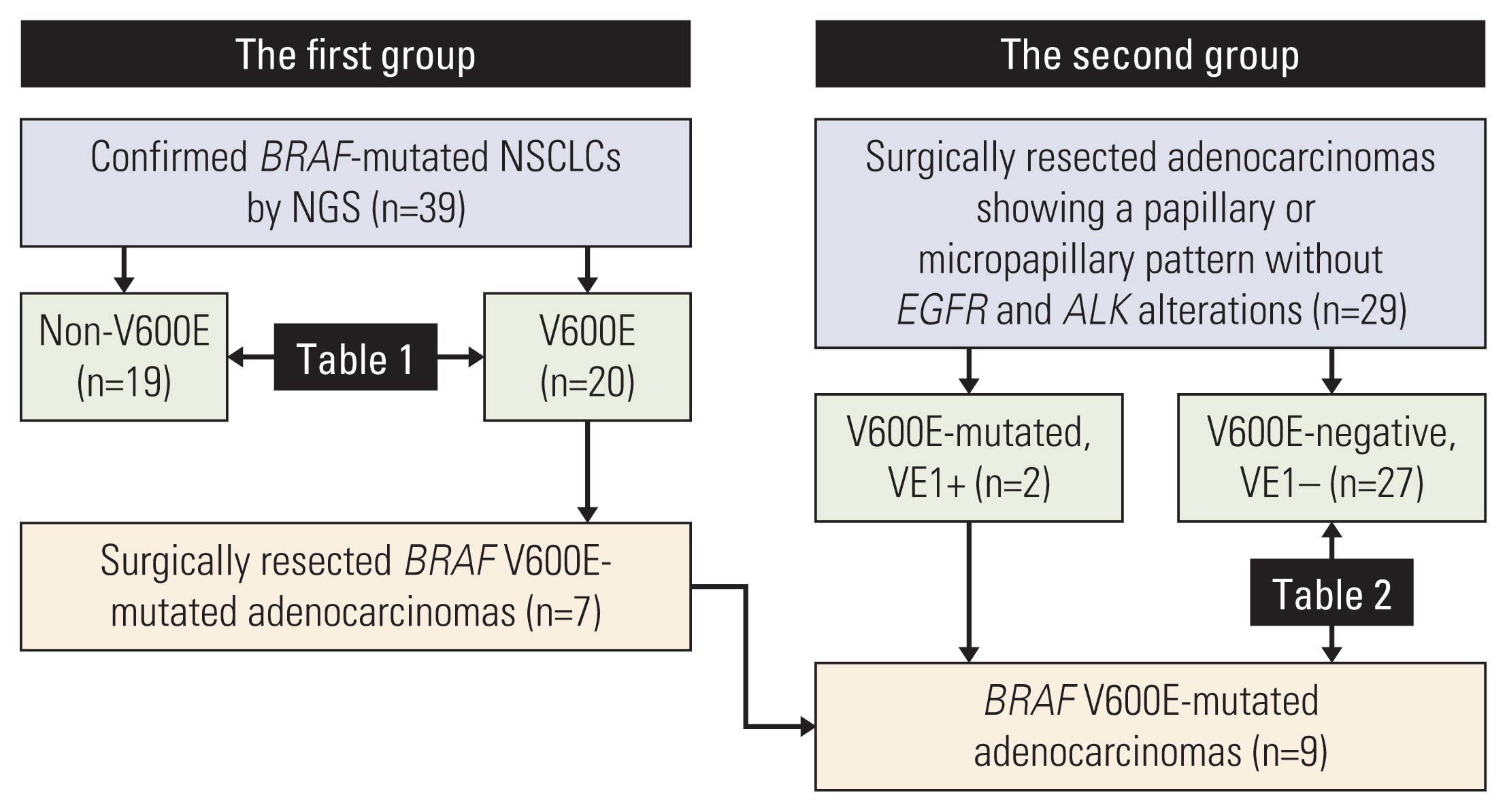
The study was designed into two steps. In a first group, BRAF-mutated NSCLCs were identified from sequencing data to determine the features of BRAF V600E mutation. The results of the first group helped the collection of adenocarcinomas with a papillary or micropapillary pattern but without EGFR or ALK alterations as a second group so that the frequency of BRAF V600E mutation could be calculated. The sensitivity and specificity of the VE1 were compared with BRAF V600E status.
Results
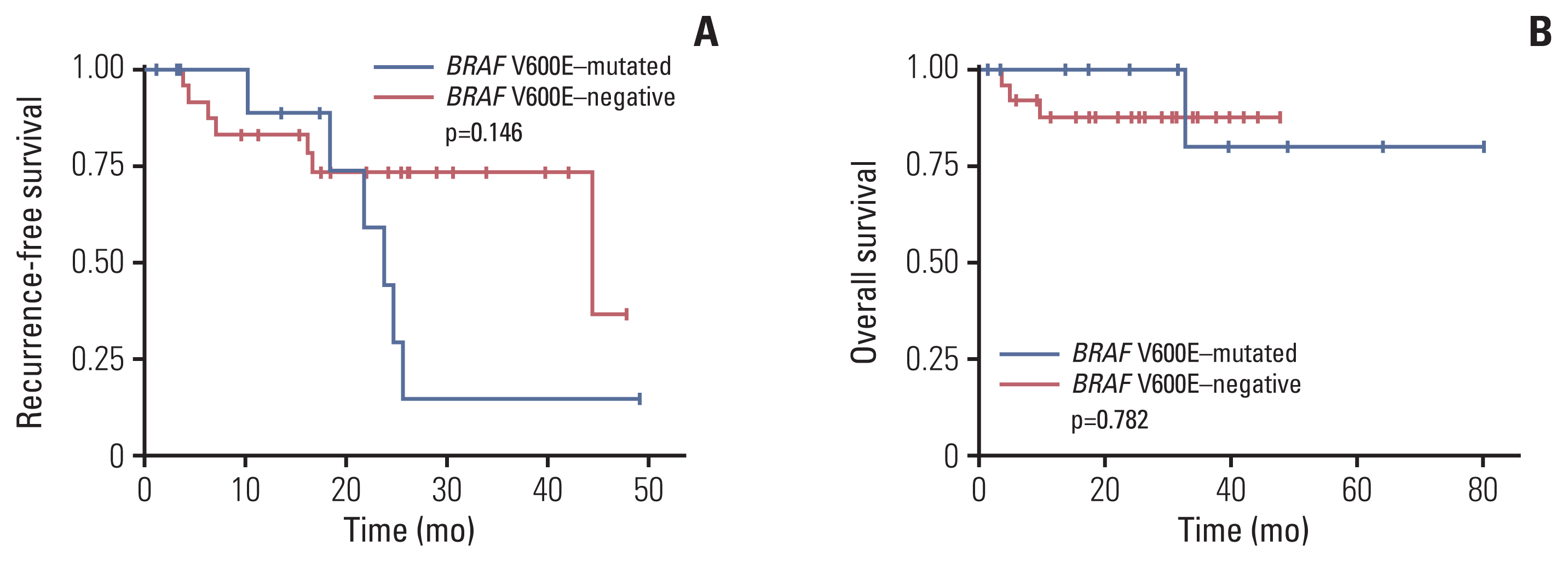
Among 39 BRAF-mutated NSCLCs in the first group, 20 (51%) were V600E. BRAF V600E mutation was more common in female patients and showed no significant correlation with smoking status. Nineteen cases were adenocarcinomas without EGFR and ALK alterations. The most common patterns of invasion were papillary and micropapillary along with central fibrosis. The sensitivity and specificity of the VE1 were 90.0% and 92.3%, respectively. In the second group, 6.7% of cases were VE1-positive, indicating that the prevalence was significantly higher than that reported in previous studies (0.3-1.8%).
Conclusion
BRAF V600E-mutated NSCLCs could be enriched with the application of clinicopathologic parameters, which are not perfect. Therefore, additional VE1 immunohistochemistry may be useful as a screening method.
Figure
Cited by 1 articles
-
Usefulness of BRAF VE1 immunohistochemistry in non–small cell lung cancers: a multi-institutional study by 15 pathologists in Korea
Sunhee Chang, Yoon-La Choi, Hyo Sup Shim, Geon Kook Lee, Seung Yeon Ha
J Pathol Transl Med. 2022;56(6):334-341. doi: 10.4132/jptm.2022.08.22.
Reference
-
References
1. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020; 52:335–50.
Article2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021; 71:7–33.
Article3. Khunger A, Khunger M, Velcheti V. Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: clinical evidence and experience. Ther Adv Respir Dis. 2018; 12:1753466618767611.
Article4. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021; 19:254–66.5. Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N, et al. FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. Oncologist. 2018; 23:740–5.6. Ilie M, Long E, Hofman V, Dadone B, Marquette CH, Mouroux J, et al. Diagnostic value of immunohistochemistry for the detection of the BRAFV600E mutation in primary lung adenocarcinoma Caucasian patients. Ann Oncol. 2013; 24:742–8.7. Villaruz LC, Socinski MA, Abberbock S, Berry LD, Johnson BE, Kwiatkowski DJ, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer. 2015; 121:448–56.
Article8. Li S, Li L, Zhu Y, Huang C, Qin Y, Liu H, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer. 2014; 110:2812–20.
Article9. Kobayashi M, Sonobe M, Takahashi T, Yoshizawa A, Ishikawa M, Kikuchi R, et al. Clinical significance of BRAF gene mutations in patients with non-small cell lung cancer. Anticancer Res. 2011; 31:4619–23.10. Yao Z, Yaeger R, Rodrik-Outmezguine VS, Tao A, Torres NM, Chang MT, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017; 548:234–8.
Article11. Planchard D, Smit EF, Groen HJ, Mazieres J, Besse B, Helland A, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017; 18:1307–16.
Article12. Sivakumar S, Lucas FA, McDowell TL, Lang W, Xu L, Fujimoto J, et al. Genomic landscape of atypical adenomatous hyperplasia reveals divergent modes to lung adenocarcinoma. Cancer Res. 2017; 77:6119–30.
Article13. Kobayashi Y, Ambrogio C, Mitsudomi T. Ground-glass nodules of the lung in never-smokers and smokers: clinical and genetic insights. Transl Lung Cancer Res. 2018; 7:487–97.
Article14. Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011; 29:3574–9.15. Litvak AM, Paik PK, Woo KM, Sima CS, Hellmann MD, Arcila ME, et al. Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol. 2014; 9:1669–74.
Article16. Tissot C, Couraud S, Tanguy R, Bringuier PP, Girard N, Souquet PJ. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer. 2016; 91:23–8.
Article17. Cardarella S, Ogino A, Nishino M, Butaney M, Shen J, Lydon C, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013; 19:4532–40.18. Gow CH, Hsieh MS, Lin YT, Liu YN, Shih JY. Validation of immunohistochemistry for the detection of BRAF V600E-mutated lung adenocarcinomas. Cancers (Basel). 2019; 11:866.
Article19. Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011; 29:2046–51.20. Dagogo-Jack I, Martinez P, Yeap BY, Ambrogio C, Ferris LA, Lydon C, et al. Impact of BRAF mutation class on disease characteristics and clinical outcomes in BRAF-mutant lung cancer. Clin Cancer Res. 2019; 25:158–65.
Article21. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013; 24:2371–6.
Article22. Yousem SA, Nikiforova M, Nikiforov Y. The histopathology of BRAF-V600E-mutated lung adenocarcinoma. Am J Surg Pathol. 2008; 32:1317–21.
Article23. Kim HC, Kang YR, Ji W, Kim YJ, Yoon S, Lee JC, et al. Frequency and clinical features of BRAF mutations among patients with stage III/IV lung adenocarcinoma without EGFR/ALK aberrations. Onco Targets Ther. 2019; 12:6045–52.24. Suzuki K, Yokose T, Yoshida J, Nishimura M, Takahashi K, Nagai K, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000; 69:893–7.
Article25. Harle A, Salleron J, Franczak C, Dubois C, Filhine-Tressarieu P, Leroux A, et al. Detection of BRAF mutations using a fully automated platform and comparison with high resolution melting, real-time allele specific amplification, immunohistochemistry and next generation sequencing assays, for patients with metastatic melanoma. PLoS One. 2016; 11:e0153576.
Article26. Sasaki H, Shimizu S, Tani Y, Shitara M, Okuda K, Hikosaka Y, et al. Usefulness of immunohistochemistry for the detection of the BRAF V600E mutation in Japanese lung adenocarcinoma. Lung Cancer. 2013; 82:51–4.
Article27. Hofman V, Benzaquen J, Heeke S, Lassalle S, Poudenx M, Long E, et al. Real-world assessment of the BRAF status in non-squamous cell lung carcinoma using VE1 immunohistochemistry: a single laboratory experience (LPCE, Nice, France). Lung Cancer. 2020; 145:58–62.
Article28. Jones RT, Abedalthagafi MS, Brahmandam M, Greenfield EA, Hoang MP, Louis DN, et al. Cross-reactivity of the BRAF VE1 antibody with epitopes in axonemal dyneins leads to staining of cilia. Mod Pathol. 2015; 28:596–606.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination of Dabrafenib and Trametinib in Patients with Metastatic BRAFV600E-Mutated Thyroid Cancer
- Clinicopathological Implications of the BRAF(V600E) Mutation in PTC with Concurrent Hashimoto Thyroiditis
- BRAF-Mutated Colorectal Cancer Exhibits Distinct Clinicopathological Features from Wild-Type BRAF-Expressing Cancer Independent of the Microsatellite Instability Status
- Clinical Implication of BRAF Mutation in Thyroid Cancer
- Small Cell Transformation in Pancreatic Metastasis from EGFR-Mutated Lung Adenocarcinoma Following TKI