J Korean Neurosurg Soc.
2022 Mar;65(2):186-195. 10.3340/jkns.2021.0082.
Histological Changes of Cervical Disc Tissue in Patients with Degenerative Ossification
- Affiliations
-
- 1School of Materials Science and Engineering, Tsinghua University, Beijing, China
- 2Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 3Department of Traditional Chinese Medicine, Peking Union Medical College Hospital, Beijing, China
- 4School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- KMID: 2527175
- DOI: http://doi.org/10.3340/jkns.2021.0082
Abstract
Objective
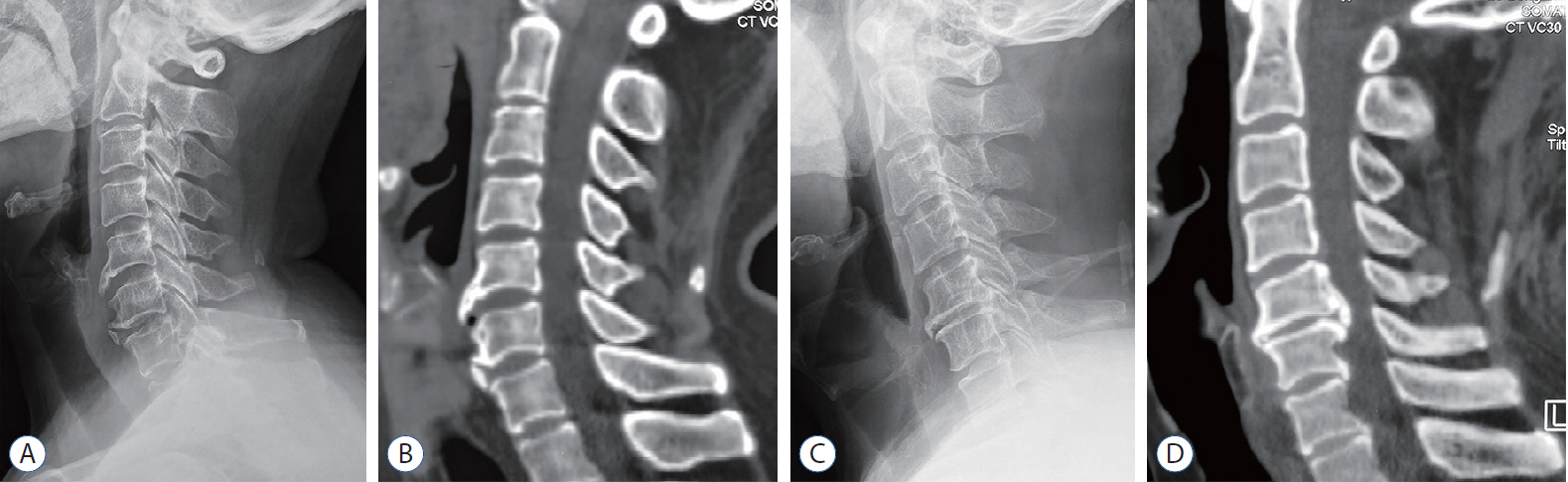
: To explore the histological feature of the cervical disc degeneration in patients with degenerative ossification (DO) and its potential mechanisms.
Methods
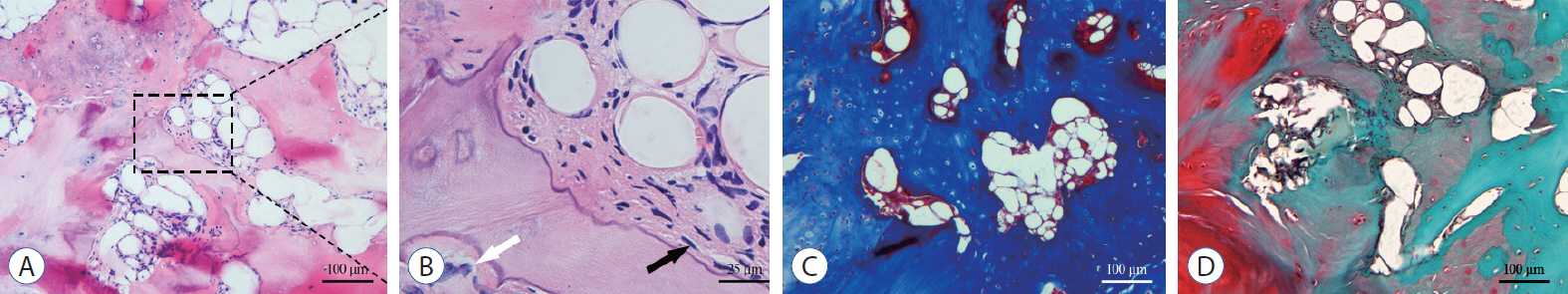
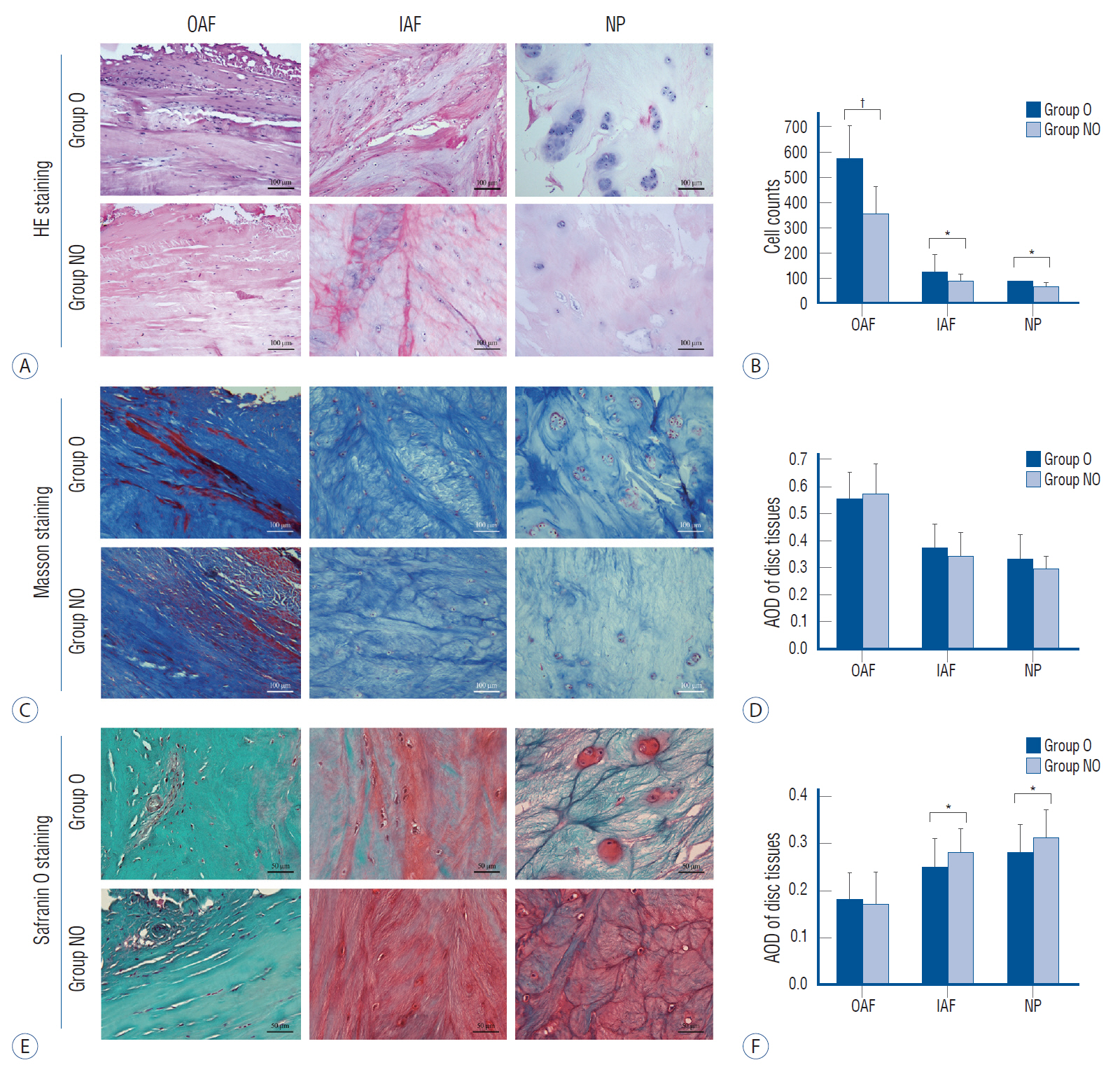
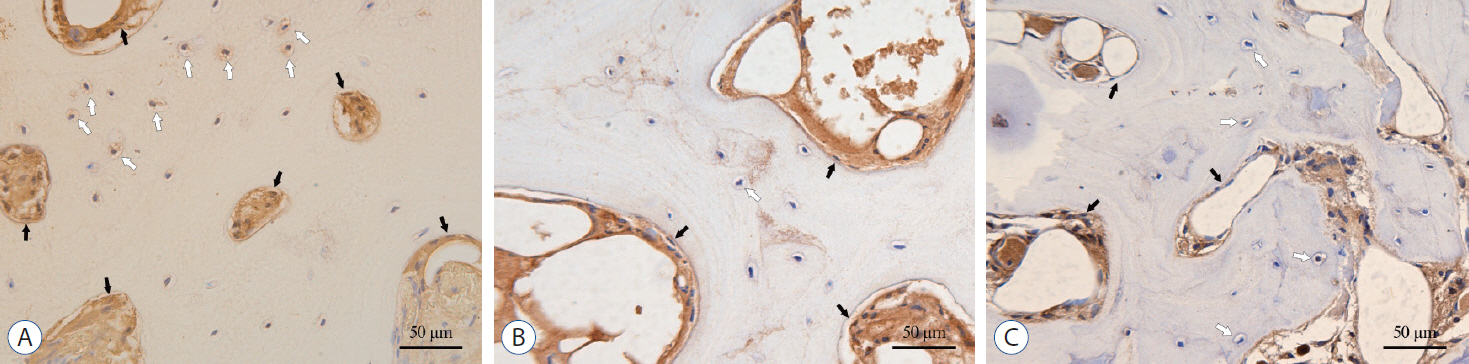
: A total of 96 surgical segments, from cervical disc degenerative disease patients with surgical treatment, were divided into ossification group (group O, n=46) and non-ossification group (group NO, n=50) based on preoperative radiological exams. Samples of disc tissues and osteophytes were harvested during the decompression operation. The hematoxylin-eosin staining, Masson trichrome staining and Safranin O-fast green staining were used to compare the histological differences between the two groups. And the distribution and content of transforming growth factor (TGF)-β1, p-Smad2 and p-Smad3 between the two groups were compared by a semi-quantitative immunohistochemistry (IHC) method.
Results
: For all the disc tissues, the content of disc cells and collagen fibers decreased gradually from the outer annulus fibrosus (OAF) to the central nucleus pulposus (NP). Compared with group NO, the number of disc cells in group O increased significantly. But for proteoglycan in the inner annulus fibrosus (IAF) and NP, the content in group O decreased significantly. IHC analysis showed that TGF-β1, p-Smad2, and p-Smad3 were detected in all tissues. For group O, the content of TGF-β1 in the OAF and NP was significantly higher than that in group NO. For p-Smad2 in IAF and p-Smad3 in OAF, the content in group O were significantly higher than group NO.
Conclusion
: Histologically, cervical disc degeneration in patients with DO is more severe than that without DO. Local higher content of TGF-β1, p-Smad2, and p-Smad3 are involved in the disc degeneration with DO. Further studies with multi-approach analyses are needed to better understand the role of TGF-β/Smads signaling pathway in the disc degeneration with DO.
Keyword
Figure
Reference
-
References
1. Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 98:996–1003. 1996.
Article2. Choi H, Tessier S, Silagi ES, Kyada R, Yousefi F, Pleshko N, et al. A novel mouse model of intervertebral disc degeneration shows altered cell fate and matrix homeostasis. Matrix Biol. 70:102–122. 2018.
Article3. Christ B, Wilting J. From somites to vertebral column. Ann Anat. 174:23–32. 1992.
Article4. Cucchiarini M, Asen AK, Goebel L, Venkatesan JK, Schmitt G, Zurakowski D, et al. Effects of TGF-β overexpression via rAAV gene transfer on the early repair processes in an osteochondral defect model in minipigs. Am J Sports Med. 46:1987–1996. 2018.
Article5. Dowdell J, Erwin M, Choma T, Vaccaro A, Iatridis J, Cho SK. Intervertebral disk degeneration and repair. Neurosurgery. 80(3S):S46–S54. 2017.
Article6. Gorth DJ, Shapiro IM, Risbud MV. A New Understanding of the Role of IL-1 in Age-Related Intervertebral Disc Degeneration in a Murine Model. J Bone Miner Res. 34:1531–1542. 2019.
Article7. Gullbrand SE, Malhotra NR, Schaer TP, Zawacki Z, Martin JT, Bendigo JR, et al. A large animal model that recapitulates the spectrum of human intervertebral disc degeneration. Osteoarthritis Cartilage. 25:146–156. 2017.
Article8. Hu Y, Tang JS, Hou SX, Shi XX, Qin J, Zhang TS, et al. Neuroprotective effects of curcumin alleviate lumbar intervertebral disc degeneration through regulating the expression of iNOS, COX-2, TGF-β1/2, MMP-9 and BDNF in a rat model. Mol Med Rep. 16:6864–6869. 2017.
Article9. Ireland D. Molecular mechanisms involved in intervertebral disc degeneration and potential new treatment strategies. Bioscience Horizons. 2:83–89. 2009.
Article10. Jin H, Shen J, Wang B, Wang M, Shu B, Chen D. TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. Febs Lett. 585:1209–1215. 2011.
Article11. Jin L, Balian G, Li XJ. Animal models for disc degeneration-an update. Histol Histopathol. 33:543–554. 2018.12. Kawaguchi Y, Furushima K, Sugimori K, Inoue I, Kimura T. Association between polymorphism of the transforming growth factor-β1 gene with the radiologic characteristic and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 28:1424–1426. 2003.
Article13. Kretschmer A, Moepert K, Dames S, Sternberger M, Kaufmann J, Klippel A. Differential regulation of TGF-beta signaling through Smad2, Smad3 and Smad4. Oncogene. 22:6748–6763. 2003.
Article14. Lawson LY, Harfe BD. Developmental mechanisms of intervertebral disc and vertebral column formation. Wiley Interdiscip Rev Dev Biol. 6:e283. 2017.
Article15. Li N, Xiu L, Guan T, Hu Z, Jin Q. Expressions of transforming growth factor β1 and connective tissue growth factor in human lumbar intervertebral discs in different degrees of degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 28:891–895. 2014.16. Li TF, O'Keefe RJ, Chen D. TGF-beta signaling in chondrocytes. Front Biosci. 10:681–688. 2005.17. Li Y, Zou N, Wang J, Wang KW, Li FY, Chen FX, et al. TGF-β1/Smad3 signaling pathway mediates T-2 toxin-induced decrease of type II collagen in cultured rat chondrocytes. Toxins (Basel). 9:359. 2017.
Article18. Nam DC, Lee HJ, Lee CJ, Hwang SC. Molecular pathophysiology of ossification of the posterior longitudinal ligament (OPLL). Biomol Ther (Seoul). 27:342–348. 2019.
Article19. Peck SH, McKee KK, Tobias JW, Malhotra NR, Harfe BD, Smith LJ. Whole transcriptome analysis of notochord-derived cells during embryonic formation of the nucleus pulposus. Sci Rep. 7:10504. 2017.
Article20. Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGF-beta signaling are essential for tendon formation. Development. 136:1351–1361. 2009.
Article21. Qian J, Ge J, Yan Q, Wu C, Yang H, Zou J. Selection of the optimal puncture needle for induction of a rat intervertebral disc degeneration model. Pain Physician. 22:353–360. 2019.22. Rutges JP, Duit RA, Kummer JA, Bekkers JE, Oner FC, Castelein RM, et al. A validated new histological classification for intervertebral disc degeneration. Osteoarthritis Cartilage. 21:2039–2047. 2013.
Article23. Sampara P, Banala RR, Vemuri SK, Av GR, Gpv S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 25:67–82. 2018.
Article24. Specchia N, Pagnotta A, Toesca A, Greco F. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 11:145–151. 2002.
Article25. Tolonen J, Grönblad M, Virri J, Seitsalo S, Rytömaa T, Karaharju E. Transforming growth factor beta receptor induction in herniated intervertebral disc tissue: an immunohistochemical study. Eur Spine J. 10:172–176. 2001.
Article26. Wang SL, Yu YL, Tang CL, Lv FZ. Effects of TGF-β1 and IL-1β on expression of ADAMTS enzymes and TIMP-3 in human intervertebral disc degeneration. Exp Ther Med. 6:1522–1526. 2013.
Article27. Williams S, Alkhatib B, Serra R. Development of the axial skeleton and intervertebral disc. Curr Top Dev Biol. 133:49–90. 2019.
Article28. Wu B, Meng C, Wang H, Jia C, Zhao Y. Changes of proteoglycan and collagen II of the adjacent intervertebral disc in the cervical instability models. Biomed Pharmacother. 84:754–758. 2016.
Article29. Yang Y, He X, Li Y, Feng J, Pang H, Wang J, et al. Association of transforming growth factor-β1 with pathological grading of intervertebral disc degeneration. Nan Fang Yi Ke Da Xue Xue Bao. 32:897–900. 2012.30. Yu ZG, Xu N, Wang WB, Pan SH, Li KS, Liu JK. Interleukin-1 inhibits Sox9 and collagen type II expression via nuclear factor-kappaB in the cultured human intervertebral disc cells. Chin Med J (Engl). 122:2483–2488. 2009.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A New Classification for Cervical Ossification of the Posterior Longitudinal Ligament Based on the Coexistence of Segmental Disc Degeneration
- Prevalence of Cervical and Thoracic Spinal Disease: A Systematic Review
- Factors Affecting Adjacent Level Ossification Development after Anterior Cervical Discectomy and Fusion
- The Efficacy of the Unicortical Screw in the Anterior Cervical Fusion of the Degenerative Cervical Spine Disease
- Pathophysiology of Degenerative Disc Disease