Int J Stem Cells.
2021 Feb;14(1):103-111. 10.15283/ijsc20078.
Culturing at Low Cell Density Delays Cellular Senescence of Human Bone Marrow-Derived Mesenchymal Stem Cells in Long-Term Cultures
- Affiliations
-
- 1SCM Lifesciences Co. Ltd., Incheon, Korea
- 2Department of Biomedical Sciences, Inha University College of Medicine, Incheon, Korea
- KMID: 2513087
- DOI: http://doi.org/10.15283/ijsc20078
Abstract
- Background and Objectives
Mesenchymal stem cells (MSCs) have immense therapeutic potential for treating intractable and immune diseases. They also have applications in regenerative medicine in which distinct treatments do not exist. Thus, MSCs are gaining attention as important raw materials in the field of cell therapy. Importantly, the number of MSCs in the bone marrow is limited and they are present only in small quantities. Therefore, mass production of MSCs through long-term culture is necessary to use them in cell therapy. However, MSCs undergo cellular senescence through repeated passages during mass production. In this study, we explored methods to prolong the limited lifetime of MSCs by culturing them with different seeding densities.
Methods and Results
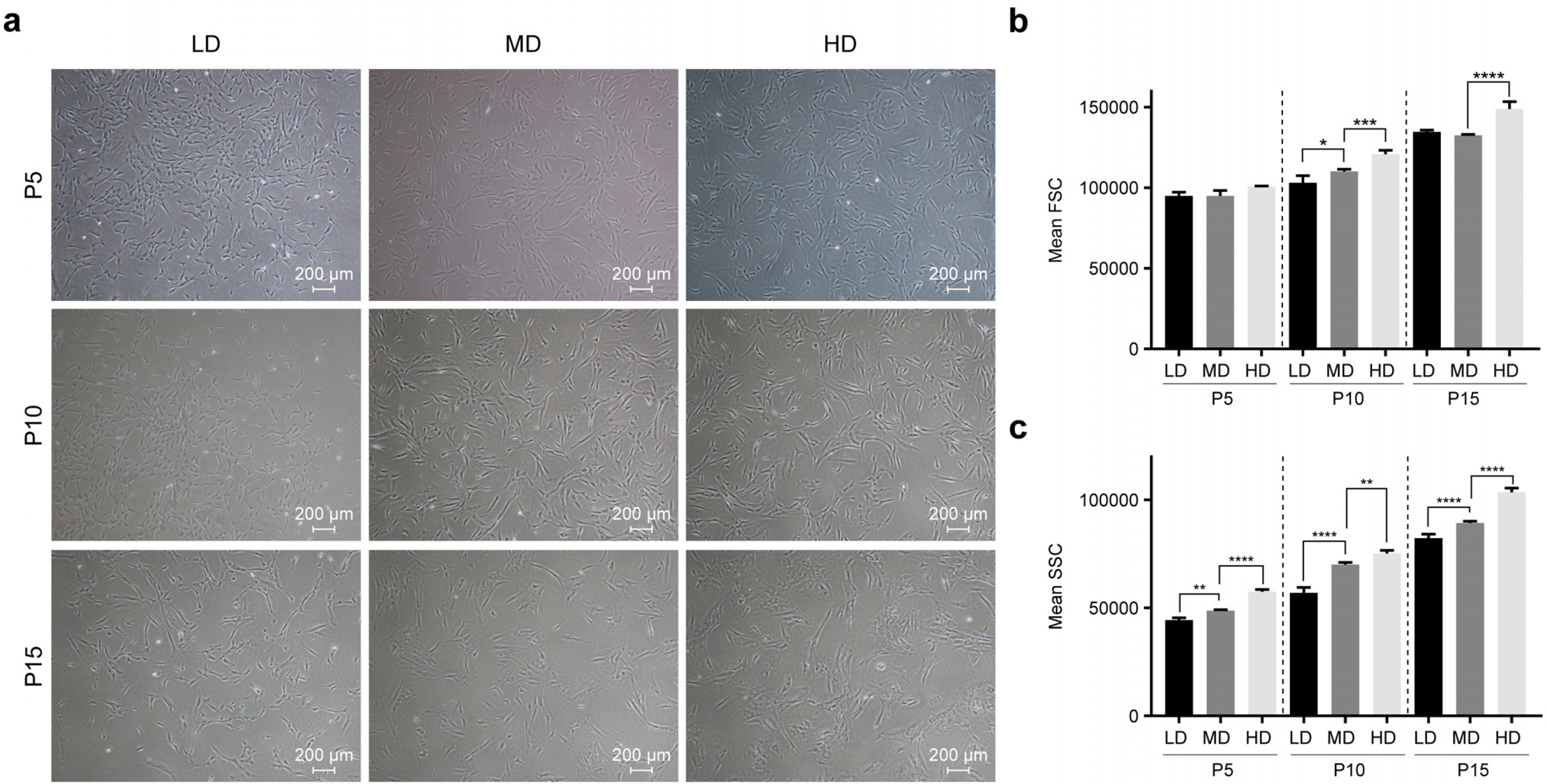
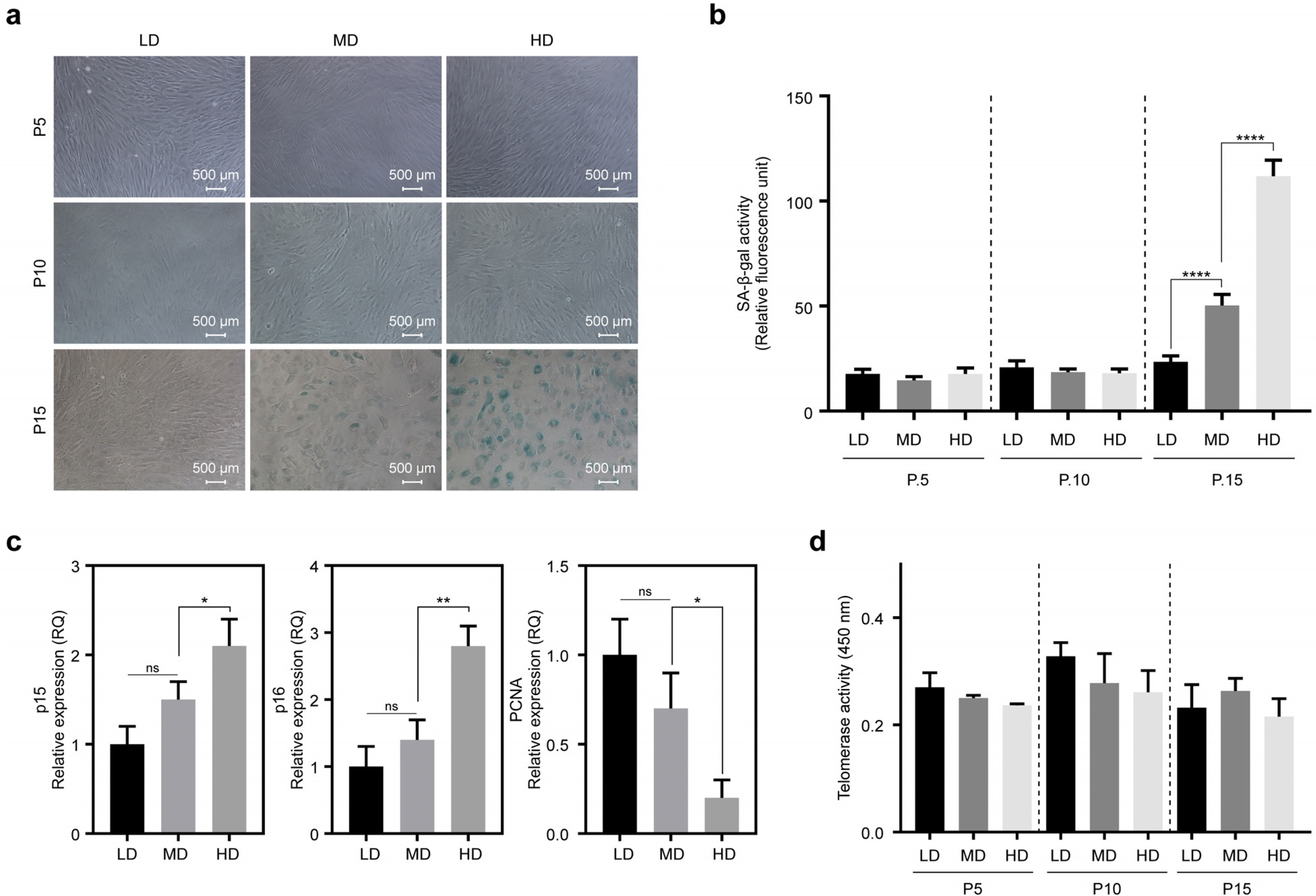
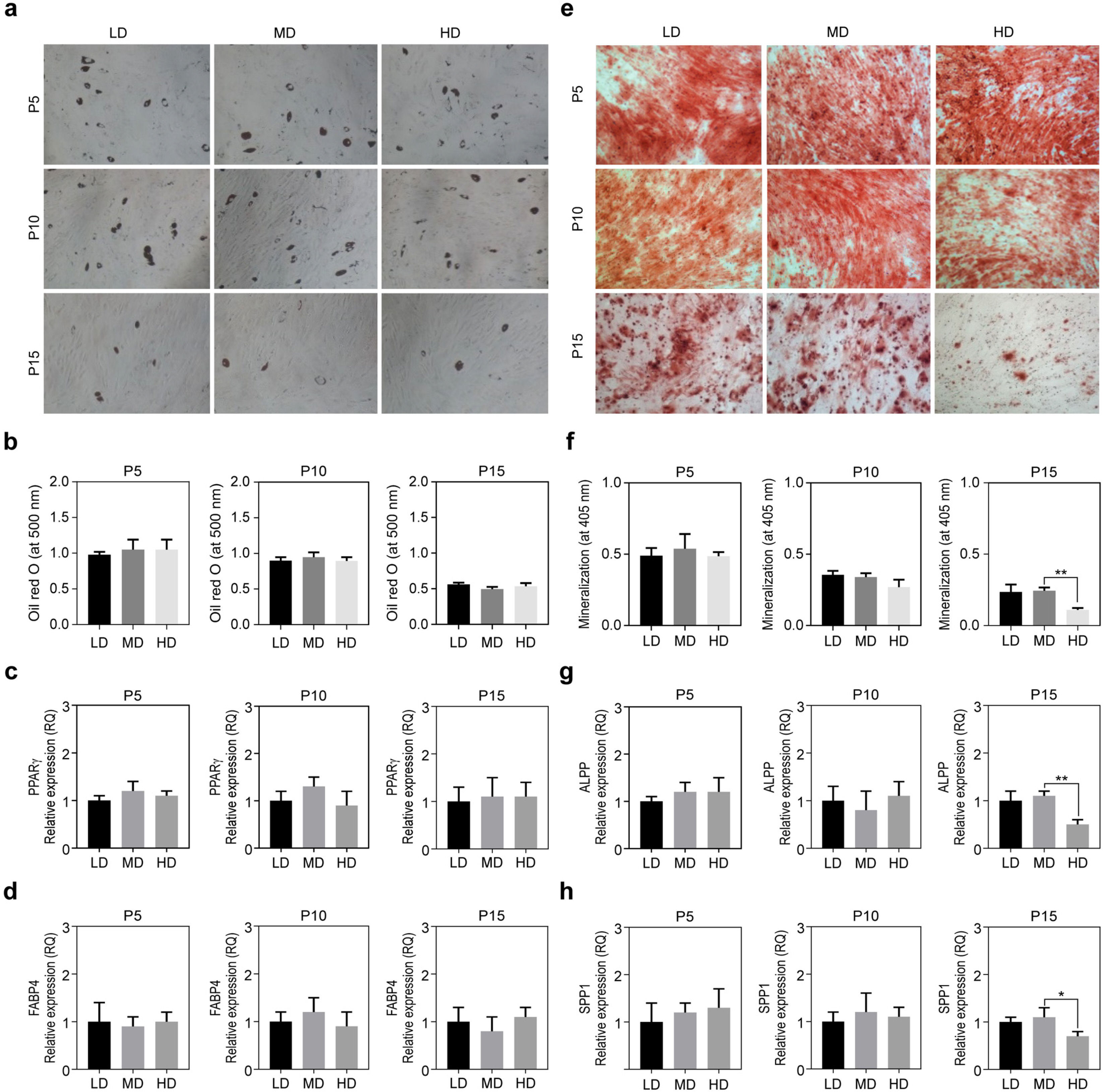
We observed that in long-term cultures, low-density (LD, 50 cells/cm2) MSCs showed higher population doubling level, leading to greater fold increase, than high-density (HD, 4,000 cells/cm2) MSCs. LD-MSCs suppressed the expression of aging-related genes. We also showed that reactive oxygen species (ROS) were decreased in LD-MSCs compared to that in HD-MSCs. Further, proliferation potential increased when ROS were inhibited in HD-MSCs.
Conclusions
The results in this study suggest that MSC senescence can be delayed and that life span can be extended by controlling cell density in vitro. These results can be used as important data for the mass production of stem cell therapeutic products.
Keyword
Figure
Reference
-
References
1. Both SK, van der Muijsenberg AJ, van Blitterswijk CA, de Boer J, de Bruijn JD. 2007; A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 13:3–9. DOI: 10.1089/ten.2005.0513. PMID: 17518576.
Article2. Koller MR, Emerson SG, Palsson BO. 1993; Large-scale expansion of human stem and progenitor cells from bone marrow mononuclear cells in continuous perfusion cultures. Blood. 82:378–384. DOI: 10.1182/blood.V82.2.378.378. PMID: 8329697.
Article3. Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, Yen BL. 2016; Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 23:76. DOI: 10.1186/s12929-016-0289-5. PMID: 27809910. PMCID: PMC5095977.
Article4. Gharibi B, Farzadi S, Ghuman M, Hughes FJ. 2014; Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells. 32:2256–2266. DOI: 10.1002/stem.1709. PMID: 24659476.
Article5. de Witte SFH, Lambert EE, Merino A, Strini T, Douben HJCW, O'Flynn L, Elliman SJ, de Klein AJEMM, Newsome PN, Baan CC, Hoogduijn MJ. 2017; Aging of bone marrow- and umbilical cord-derived mesenchymal stromal cells during expansion. Cytotherapy. 19:798–807. DOI: 10.1016/j.jcyt.2017.03.071. PMID: 28462821.
Article6. Sethe S, Scutt A, Stolzing A. 2006; Aging of mesenchymal stem cells. Ageing Res Rev. 5:91–116. DOI: 10.1016/j.arr.2005.10.001. PMID: 16310414.
Article7. Basciano L, Nemos C, Foliguet B, de Isla N, de Carvalho M, Tran N, Dalloul A. 2011; Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 12:12. DOI: 10.1186/1471-2121-12-12. PMID: 21450070. PMCID: PMC3073900.
Article8. Ejtehadifar M, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P, Molaeipour Z, Saleh M. 2015; The effect of hypoxia on mesenchymal stem cell biology. Adv Pharm Bull. 5:141–149. DOI: 10.15171/apb.2015.021. PMID: 26236651. PMCID: PMC4517092.
Article9. Khan M, Akhtar S, Mohsin S, N Khan S, Riazuddin S. 2011; Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 20:67–75. DOI: 10.1089/scd.2009.0397. PMID: 20446810.
Article10. Kim JH, Kim WK, Sung YK, Kwack MH, Song SY, Choi JS, Park SG, Yi T, Lee HJ, Kim DD, Seo HM, Song SU, Sung JH. 2014; The molecular mechanism underlying the proliferating and preconditioning effect of vitamin C on adipose-derived stem cells. Stem Cells Dev. 23:1364–1376. DOI: 10.1089/scd.2013.0460. PMID: 24524758. PMCID: PMC4046194.
Article11. Rodrigues M, Griffith LG, Wells A. 2010; Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 1:32. DOI: 10.1186/scrt32. PMID: 20977782. PMCID: PMC2983445.
Article12. Misra J, Mohanty ST, Madan S, Fernandes JA, Hal Ebetino F, Russell RG, Bellantuono I. 2016; Zoledronate attenuates accumulation of DNA damage in mesenchymal stem cells and protects their function. Stem Cells. 34:756–767. DOI: 10.1002/stem.2255. PMID: 26679354. PMCID: PMC4832316.
Article13. Oh J, Lee YD, Wagers AJ. 2014; Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 20:870–880. DOI: 10.1038/nm.3651. PMID: 25100532. PMCID: PMC4160113.
Article14. Schultz MB, Sinclair DA. 2016; When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 143:3–14. DOI: 10.1242/dev.130633. PMID: 26732838. PMCID: PMC4725211.
Article15. Kim DS, Lee MW, Ko YJ, Chun YH, Kim HJ, Sung KW, Koo HH, Yoo KH. 2016; Cell culture density affects the proliferation activity of human adipose tissue stem cells. Cell Biochem Funct. 34:16–24. DOI: 10.1002/cbf.3158. PMID: 26778408.
Article16. Kim DS, Lee MW, Yoo KH, Lee TH, Kim HJ, Jang IK, Chun YH, Kim HJ, Park SJ, Lee SH, Son MH, Jung HL, Sung KW, Koo HH. 2014; Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One. 9:e83363. DOI: 10.1371/journal.pone.0083363. PMID: 24400072. PMCID: PMC3882209.
Article17. Kim DS, Lee MW, Lee TH, Sung KW, Koo HH, Yoo KH. 2017; Cell culture density affects the stemness gene expression of adipose tissue-derived mesenchymal stem cells. Biomed Rep. 6:300–306. DOI: 10.3892/br.2017.845. PMID: 28451390. PMCID: PMC5403436.
Article18. Jeon MS, Yi TG, Lim HJ, Moon SH, Lee MH, Kang JS, Kim CS, Lee DH, Song SU. 2011; Characterization of mouse clonal mesenchymal stem cell lines established by subfrac-tionation culturing method. World J Stem Cells. 3:70–82. DOI: 10.4252/wjsc.v3.i8.70. PMID: 22007272. PMCID: PMC3192225.
Article19. Signer RA, Morrison SJ. 2013; Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 12:152–165. DOI: 10.1016/j.stem.2013.01.001. PMID: 23395443. PMCID: PMC3641677.
Article20. Zhao Q, Wang XY, Yu XX, Zhai YX, He X, Wu S, Shi YA. 2015; Expression of human telomerase reverse transcriptase mediates the senescence of mesenchymal stem cells through the PI3K/AKT signaling pathway. Int J Mol Med. 36:857–864. DOI: 10.3892/ijmm.2015.2284. PMID: 26178664.
Article21. Zimmermann S, Voss M, Kaiser S, Kapp U, Waller CF, Martens UM. 2003; Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 17:1146–1149. DOI: 10.1038/sj.leu.2402962. PMID: 12764382.
Article22. Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. 2008; Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 3:e2213. DOI: 10.1371/journal.pone.0002213. PMID: 18493317. PMCID: PMC2374903.
Article23. Feng G, Tan W, Gu Z. 2013; Mesenchymal stem cells and senescence. Clon Transgen. 2:104. DOI: 10.4172/2168-9849.1000104.
Article24. Jacobs K, Zambelli F, Mertzanidou A, Smolders I, Geens M, Nguyen HT, Barbé L, Sermon K, Spits C. 2016; Higher-density culture in human embryonic stem cells results in DNA damage and genome instability. Stem Cell Reports. 6:330–341. DOI: 10.1016/j.stemcr.2016.01.015. PMID: 26923824. PMCID: PMC4788786.
Article25. Yang SR, Park JR, Kang KS. 2015; Reactive oxygen species in mesenchymal stem cell aging: implication to lung diseases. Oxid Med Cell Longev. 2015:486263. DOI: 10.1155/2015/486263. PMID: 26273422. PMCID: PMC4529978.
Article26. Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. 2017; Senescence of mesenchymal stem cells (Review). Int J Mol Med. 39:775–782. DOI: 10.3892/ijmm.2017.2912. PMID: 28290609.
Article27. Denu RA, Hematti P. 2016; Effects of oxidative stress on mesenchymal stem cell biology. Oxid Med Cell Longev. 2016:2989076. DOI: 10.1155/2016/2989076. PMID: 27413419. PMCID: PMC4928004.
Article28. Vono R, Jover Garcia E, Spinetti G, Madeddu P. 2018; Oxidative stress in mesenchymal stem cell senescence: regulation by coding and noncoding RNAs. Antioxid Redox Signal. 29:864–879. DOI: 10.1089/ars.2017.7294. PMID: 28762752. PMCID: PMC6080119.
Article29. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. 2013; Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 34:747–754. DOI: 10.1038/aps.2013.50. PMID: 23736003. PMCID: PMC4002895.
Article30. Bartmann C, Rohde E, Schallmoser K, Pürstner P, Lanzer G, Linkesch W, Strunk D. 2007; Two steps to functional mesenchymal stromal cells for clinical application. Transfusion. 47:1426–1435. DOI: 10.1111/j.1537-2995.2007.01219.x. PMID: 17655587.
Article31. Colter DC, Class R, DiGirolamo CM, Prockop DJ. 2000; Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 97:3213–3218. DOI: 10.1073/pnas.97.7.3213. PMID: 10725391. PMCID: PMC16218.
Article32. Fekete N, Rojewski MT, Fürst D, Kreja L, Ignatius A, Dausend J, Schrezenmeier H. 2012; GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 7:e43255. DOI: 10.1371/journal.pone.0043255. PMID: 22905242. PMCID: PMC3419200.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nervonic Acid Inhibits Replicative Senescence of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells
- Characterization of Senescence of Culture-expanded Human Adipose-derived Mesenchymal Stem Cells
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Evaluation of Bone Marrow-derived Stem Cells and Adipose-derived Stem Cells Co-cultured on Human Nucleus Pulposus Cells: A Pilot Study