Int J Stem Cells.
2024 Feb;17(1):80-90. 10.15283/ijsc23101.
Nervonic Acid Inhibits Replicative Senescence of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells
- Affiliations
-
- 1Cell and Gene Therapy Institute, ENCell Co. Ltd., Seoul, Korea
- 2Cell and Gene Therapy Institute, Samsung Medical Center, Seoul, Korea
- 3Department of Obstetrics and Gynecology, Samsung Medical Center, Seoul, Korea
- 4Department of Medical Device Management and Research, SAIHST, Sungkyunkwan University, Seoul, Korea
- 5The Office of R&D Strategy & Planning, Samsung Medical Center, Seoul, Korea
- 6Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul, Korea
- KMID: 2552020
- DOI: http://doi.org/10.15283/ijsc23101
Abstract
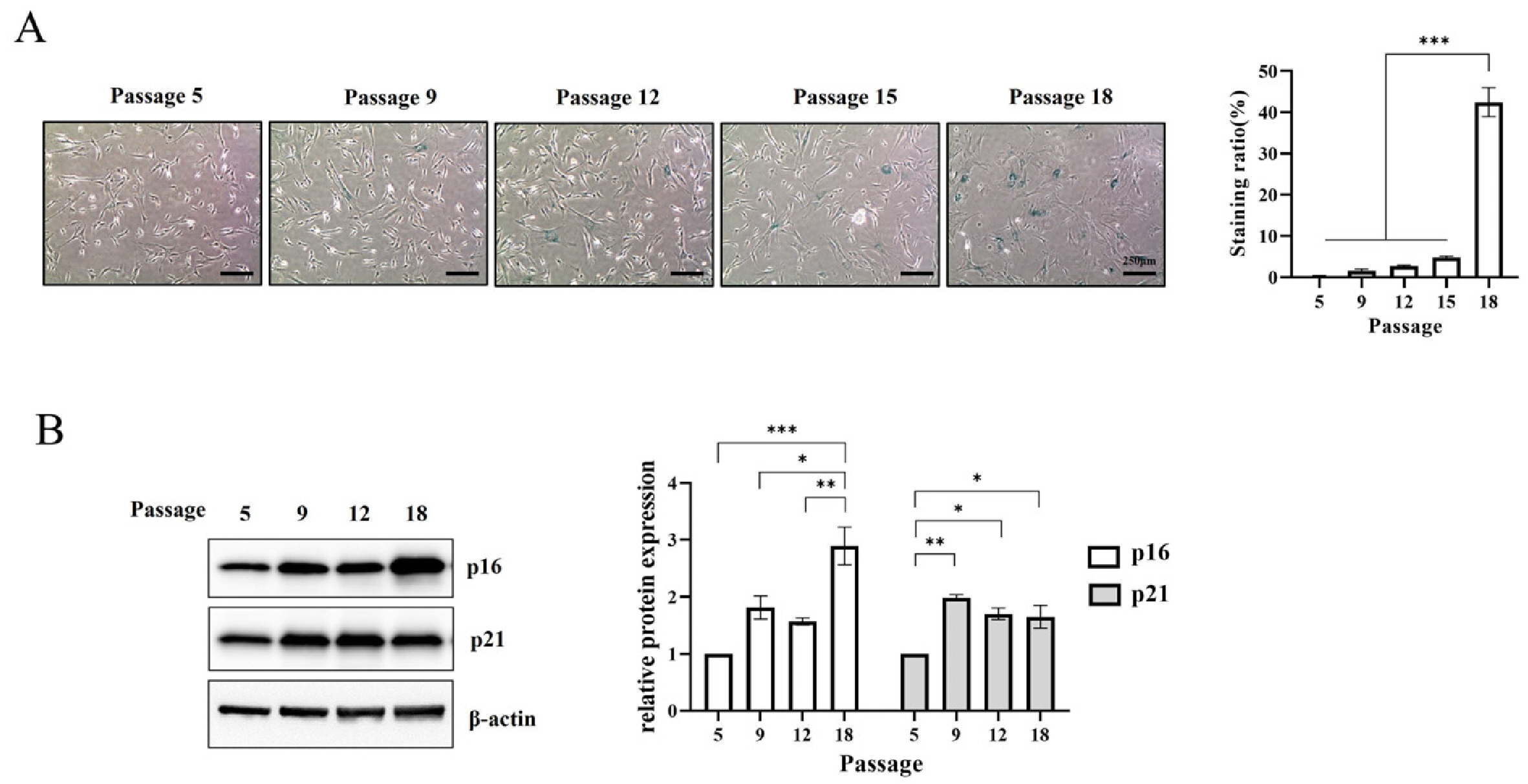
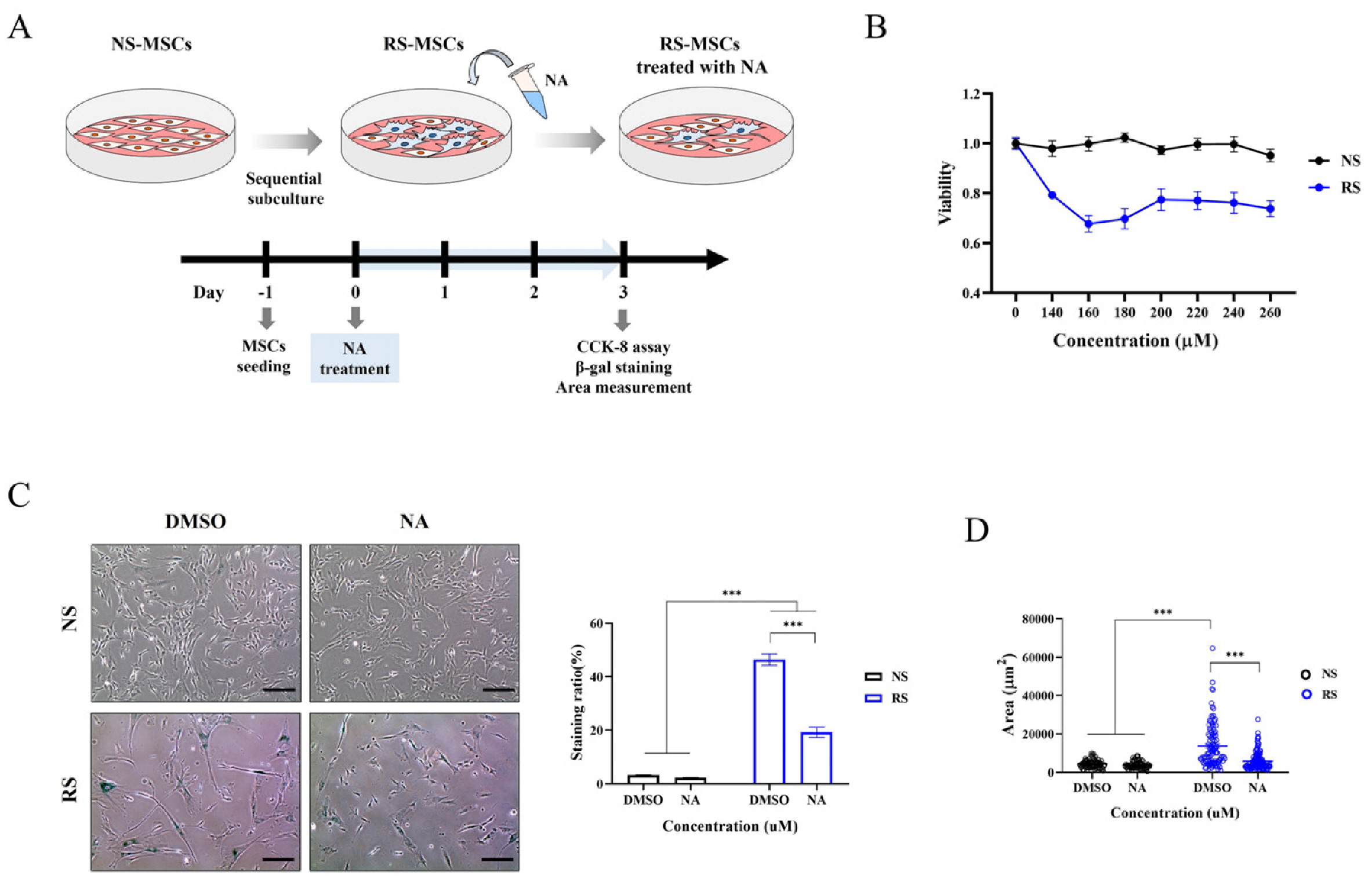
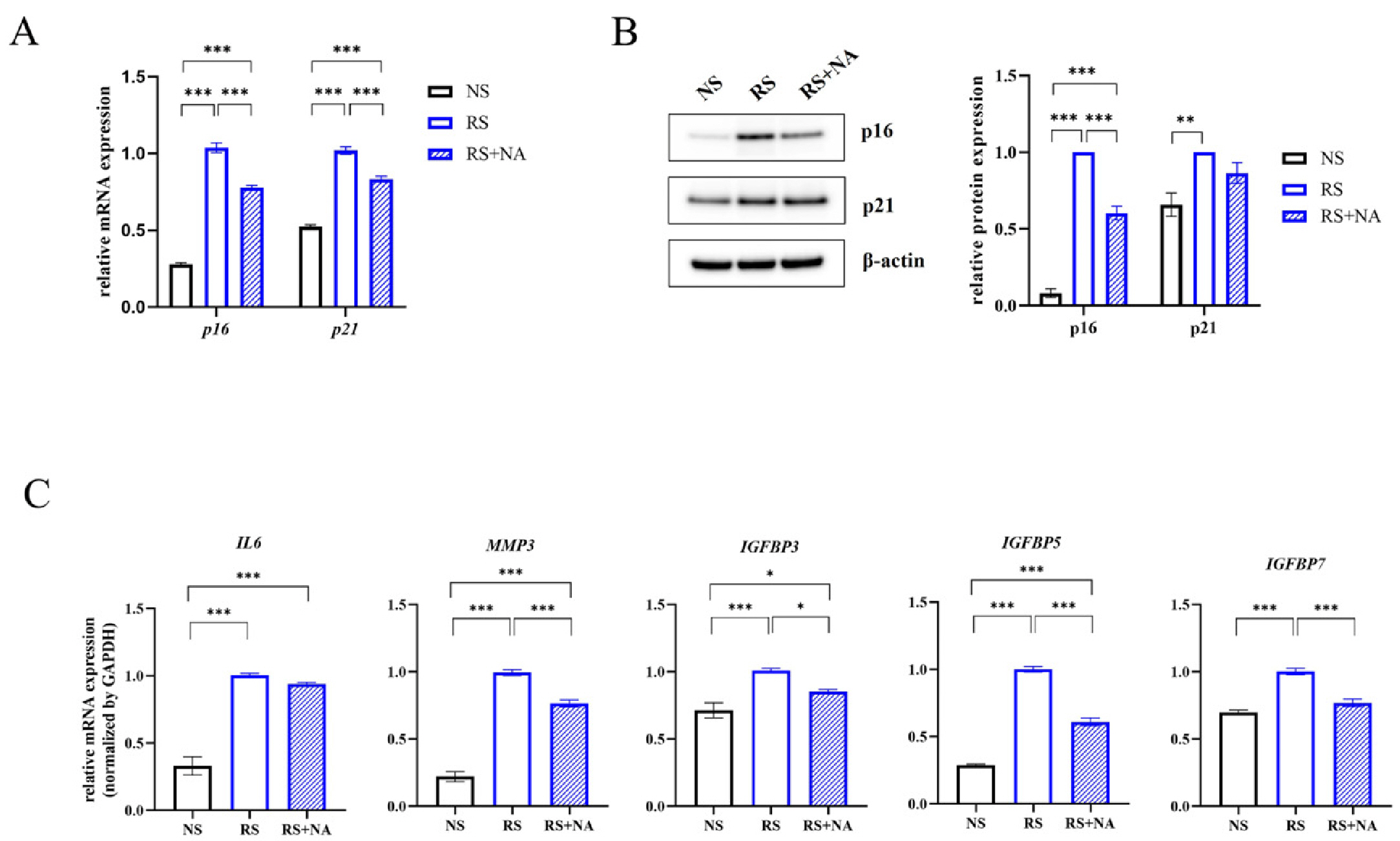
- Cellular senescence causes cell cycle arrest and promotes permanent cessation of proliferation. Since the senescence of mesenchymal stem cells (MSCs) reduces proliferation and multipotency and increases immunogenicity, aged MSCs are not suitable for cell therapy. Therefore, it is important to inhibit cellular senescence in MSCs. It has recently been reported that metabolites can control aging diseases. Therefore, we aimed to identify novel metabolites that regulate the replicative senescence in MSCs. Using a fecal metabolites library, we identified nervonic acid (NA) as a candidate metabolite for replicative senescence regulation. In replicative senescent MSCs, NA reduced senescence-associated β-galactosidase positive cells, the expression of senescence-related genes, as well as increased stemness and adipogenesis. Moreover, in non-senescent MSCs, NA treatment delayed senescence caused by sequential subculture and promoted proliferation. We confirmed, for the first time, that NA delayed and inhibited cellular senescence. Considering optimal concentration, duration, and timing of drug treatment, NA is a novel potential metabolite that can be used in the development of technologies that regulate cellular senescence.
Figure
Reference
-
References
1. Hernandez-Segura A, Nehme J, Demaria M. 2018; Hallmarks of cellular senescence. Trends Cell Biol. 28:436–453. DOI: 10.1016/j.tcb.2018.02.001. PMID: 29477613.
Article2. Kim SJ, Park SE, Jeong JB, et al. 2022; Wharton's jelly-derived mesenchymal stem cells with high aurora kinase A expression show improved proliferation, migration, and therapeutic potential. Stem Cells Int. 2022:4711499. DOI: 10.1155/2022/4711499. PMID: 35450345. PMCID: PMC9017458. PMID: acf4e35b797c4df39fe1246d49f773c3.
Article3. Weng Z, Wang Y, Ouchi T, et al. 2022; Mesenchymal stem/stromal cell senescence: hallmarks, mechanisms, and combating strategies. Stem Cells Transl Med. 11:356–371. DOI: 10.1093/stcltm/szac004. PMID: 35485439. PMCID: PMC9052415.
Article4. Zhou X, Hong Y, Zhang H, Li X. 2020; Mesenchymal stem cell senescence and rejuvenation: current status and challenges. Front Cell Dev Biol. 8:364. DOI: 10.3389/fcell.2020.00364. PMID: 32582691. PMCID: PMC7283395. PMID: 9a2677b465684f21bf7f20f2359c25b6.
Article5. Liu J, Ding Y, Liu Z, Liang X. 2020; Senescence in mesenchymal stem cells: functional alterations, molecular mechanisms, and rejuvenation strategies. Front Cell Dev Biol. 8:258. DOI: 10.3389/fcell.2020.00258. PMID: 32478063. PMCID: PMC7232554. PMID: 0fd3703ff04d4fdba0120c802d5e4b7d.
Article6. Sharma R. 2022; Emerging interrelationship between the gut microbiome and cellular senescence in the context of aging and disease: perspectives and therapeutic opportunities. Probiotics Antimicrob Proteins. 14:648–663. DOI: 10.1007/s12602-021-09903-3. PMID: 34985682. PMCID: PMC8728710.
Article7. Parker A, Romano S, Ansorge R, et al. 2022; Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 10:68. DOI: 10.1186/s40168-022-01243-w. PMID: 35501923. PMCID: PMC9063061. PMID: cb0e43327b1d4f1c8b7dc50edb1d5e97.
Article8. Zheng W, Kollmeyer J, Symolon H, et al. 2006; Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Bio-phys Acta. 1758:1864–1884. DOI: 10.1016/j.bbamem.2006.08.009. PMID: 17052686.
Article9. Li Q, Chen J, Yu X, Gao JM. 2019; A mini review of nervonic acid: source, production, and biological functions. Food Chem. 301:125286. DOI: 10.1016/j.foodchem.2019.125286. PMID: 31382110.
Article10. Yu J, Yuan T, Zhang X, Jin Q, Wei W, Wang X. 2019; Quantifi-cation of nervonic acid in human milk in the first 30 days of lactation: influence of lactation stages and comparison with infant formulae. Nutrients. 11:1892. DOI: 10.3390/nu11081892. PMID: 31416149. PMCID: PMC6723218. PMID: 1da3f5a67d444a748851331ca8d8351c.
Article11. Wu R, Zhong S, Ni M, et al. 2020; Effects of Malania oleifera Chun oil on the improvement of learning and memory fun-ction in mice. Evid Based Complement Alternat Med. 2020:8617143. DOI: 10.1155/2020/8617143. PMID: 33014116. PMCID: PMC7519201.12. Kwon S, Ki SM, Park SE, et al. 2016; Anti-apoptotic effects of human Wharton's jelly-derived mesenchymal stem cells on skeletal muscle cells mediated via secretion of XCL1. Mol Ther. 24:1550–1560. DOI: 10.1038/mt.2016.125. PMID: 27434589. PMCID: PMC5113102.
Article13. Palumbo P, Lombardi F, Siragusa G, Cifone MG, Cinque B, Giuliani M. 2018; Methods of isolation, characterization and expansion of human adipose-derived stem cells (ASCs): an overview. Int J Mol Sci. 19:1897. DOI: 10.3390/ijms19071897. PMID: 29958391. PMCID: PMC6073397. PMID: 06437a0590424434beac2ab4eb4319aa.
Article14. Choi YS, Park YB, Ha CW, et al. 2017; Different characteristics of mesenchymal stem cells isolated from different layers of full term placenta. PLoS One. 12:e0172642. DOI: 10.1371/journal.pone.0172642. PMID: 28225815. PMCID: PMC5321410. PMID: c5c5f0f5509d44469c2ec827af3db10b.
Article15. Kim JY, Kim DH, Kim DS, et al. 2010; Galectin-3 secreted by human umbilical cord blood-derived mesenchymal stem cells reduces amyloid-beta42 neurotoxicity in vitro. FEBS Lett. 584:3601–3608. DOI: 10.1016/j.febslet.2010.07.028. PMID: 20655311.
Article16. Dominici M, Le Blanc K, Mueller I, et al. 2006; Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article17. Byun HO, Lee YK, Kim JM, Yoon G. 2015; From cell senescence to age-related diseases: differential mechanisms of action of senescence-associated secretory phenotypes. BMB Rep. 48:549–558. Erratum in: BMB Rep 2016;49:641-650. DOI: 10.5483/BMBRep.2015.48.10.122. PMID: 26129674. PMCID: PMC4911181.
Article18. Kamal MM, Kassem DH. 2020; Therapeutic potential of Wharton's jelly mesenchymal stem cells for diabetes: achievements and challenges. Front Cell Dev Biol. 8:16. DOI: 10.3389/fcell.2020.00016. PMID: 32064260. PMCID: PMC7000356. PMID: c982e4038ec34dd9a4f1a4119814e739.
Article19. Neri S, Borzì RM. 2020; Molecular mechanisms contributing to mesenchymal stromal cell aging. Biomolecules. 10:340. DOI: 10.3390/biom10020340. PMID: 32098040. PMCID: PMC7072652. PMID: 13ca9f52082a4d7caf70208317e8dccb.
Article20. Li J, Han S, Cousin W, Conboy IM. 2015; Age-specific functional epigenetic changes in p21 and p16 in injury-activated satellite cells. Stem Cells. 33:951–961. DOI: 10.1002/stem.1908. PMID: 25447026. PMCID: PMC4333004.
Article21. Stein GH, Drullinger LF, Soulard A, Dulić V. 1999; Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 19:2109–2117. DOI: 10.1128/MCB.19.3.2109. PMID: 10022898. PMCID: PMC84004.
Article22. Liao Z, Yeo HL, Wong SW, Zhao Y. 2021; Cellular senescence: mechanisms and therapeutic potential. Biomedicines. 9:1769. DOI: 10.3390/biomedicines9121769. PMID: 34944585. PMCID: PMC8698401. PMID: f472d8b0e8964ce8906fccc715fc8556.
Article23. Kumari R, Jat P. 2021; Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 9:645593. DOI: 10.3389/fcell.2021.645593. PMID: 33855023. PMCID: PMC8039141. PMID: 479af44c870c4eab9f9786a4796829fe.
Article24. Chinnadurai R, Rajan D, Ng S, et al. 2017; Immune dysfunctionality of replicative senescent mesenchymal stromal cells is corrected by IFNγ priming. Blood Adv. 1:628–643. DOI: 10.1182/bloodadvances.2017006205. PMID: 28713871. PMCID: PMC5507374.
Article25. Nam K, Oh S, Lee KM, Yoo SA, Shin I. 2015; CD44 regulates cell proliferation, migration, and invasion via modulation of c-Src transcription in human breast cancer cells. Cell Signal. 27:1882–1894. DOI: 10.1016/j.cellsig.2015.05.002. PMID: 25979842.
Article26. Ludwig N, Szczepanski MJ, Gluszko A, et al. 2019; CD44(+) tumor cells promote early angiogenesis in head and neck squamous cell carcinoma. Cancer Lett. 467:85–95. DOI: 10.1016/j.canlet.2019.10.010. PMID: 31593802.
Article27. Petruk N, Tuominen S, Åkerfelt M, et al. 2021; CD73 facilitates EMT progression and promotes lung metastases in triple-negative breast cancer. Sci Rep. 11:6035. DOI: 10.1038/s41598-021-85379-z. PMID: 33727591. PMCID: PMC7966763. PMID: b2c631e7001b40c2910848b8522fac2a.
Article28. Dijk W, Kersten S. 2014; Regulation of lipoprotein lipase by Angptl4. Trends Endocrinol Metab. 25:146–155. DOI: 10.1016/j.tem.2013.12.005. PMID: 24397894.
Article29. Adhikary T, Brandt DT, Kaddatz K, et al. 2013; Inverse PPARβ/δ agonists suppress oncogenic signaling to the ANGPTL4 gene and inhibit cancer cell invasion. Oncogene. 32:5241–5252. DOI: 10.1038/onc.2012.549. PMID: 23208498. PMCID: PMC3938163.
Article30. Li X, Chen T, Shi Q, et al. 2015; Angiopoietin-like 4 enhances metastasis and inhibits apoptosis via inducing bone morphogenetic protein 7 in colorectal cancer cells. Biochem Biophys Res Commun. 467:128–134. DOI: 10.1016/j.bbrc.2015.09.104. PMID: 26417691.
Article31. Conte M, Franceschi C, Sandri M, Salvioli S. 2016; Perilipin 2 and age-related metabolic diseases: a new perspective. Trends Endocrinol Metab. 27:893–903. DOI: 10.1016/j.tem.2016.09.001. PMID: 27659144.
Article32. Attie AD, Kastelein JP, Hayden MR. 2001; Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J Lipid Res. 42:1717–1726. DOI: 10.1016/S0022-2275(20)31498-X. PMID: 11714841.
Article33. Tao H, Han Z, Han ZC, Li Z. 2016; Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int. 2016:1314709. DOI: 10.1155/2016/1314709. PMID: 26880933. PMCID: PMC4736816. PMID: 4fc577b6b0264eaba6fa7b81d84f2429.
Article34. Muppala S, Xiao R, Krukovets I, et al. 2017; Thrombospondin-4 mediates TGF-β-induced angiogenesis. Oncogene. 36:5189–5198. DOI: 10.1038/onc.2017.140. PMID: 28481870. PMCID: PMC5589494.
Article35. Zhang Q, Zhou M, Wu X, et al. 2019; Promoting therapeutic angiogenesis of focal cerebral ischemia using thrombospondin-4 (TSP4) gene-modified bone marrow stromal cells (BMSCs) in a rat model. J Transl Med. 17:111. DOI: 10.1186/s12967-019-1845-z. PMID: 30947736. PMCID: PMC6449913. PMID: 8775af588db3458ba3bf1a0c70484dff.
Article36. Isenberg JS, Roberts DD. 2020; Thrombospondin-1 in maladaptive aging responses: a concept whose time has come. Am J Physiol Cell Physiol. 319:C45–C63. DOI: 10.1152/ajpcell.00089.2020. PMID: 32374675. PMCID: PMC7468894.
Article37. Liu Y, Chen Q. 2020; Senescent mesenchymal stem cells: disease mechanism and treatment strategy. Curr Mol Biol Rep. 6:173–182. DOI: 10.1007/s40610-020-00141-0. PMID: 33816065. PMCID: PMC8011589.
Article38. Yuan SN, Wang MX, Han JL, et al. 2023; Improved colonic inflammation by nervonic acid via inhibition of NF-κB signaling pathway of DSS-induced colitis mice. Phytomedi-cine. 112:154702. DOI: 10.1016/j.phymed.2023.154702. PMID: 36764096.
Article39. Ito TK, Yokoyama M, Yoshida Y, et al. 2014; A crucial role for CDC42 in senescence-associated inflammation and athero-sclerosis. PLoS One. 9:e102186. DOI: 10.1371/journal.pone.0102186. PMID: 25057989. PMCID: PMC4109913. PMID: 86e3f27b5bf9496c946c701d32adf391.
Article40. Lee Y, Clinton J, Yao C, Chang SH. 2019; Interleukin-17D promotes pathogenicity during infection by suppressing CD8 T cell activity. Front Immunol. 10:1172. DOI: 10.3389/fimmu.2019.01172. PMID: 31244826. PMCID: PMC6562898. PMID: dd1aa3a037334dc6970113c2b919ba93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of human male germ cells from Wharton's jelly-derived mesenchymal stem cells
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- Mesenchymal Stem Cells from the Wharton's Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential
- Cardioprotective Effects of Wharton Jelly Derived Mesenchymal Stem Cell Transplantation in a Rodent Model of Myocardial Injury
- Characterization of Senescence of Culture-expanded Human Adipose-derived Mesenchymal Stem Cells